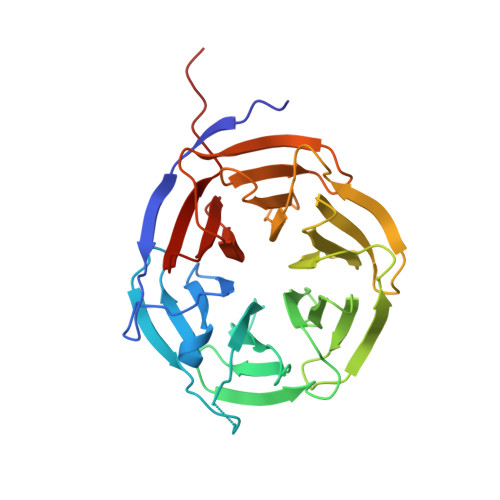
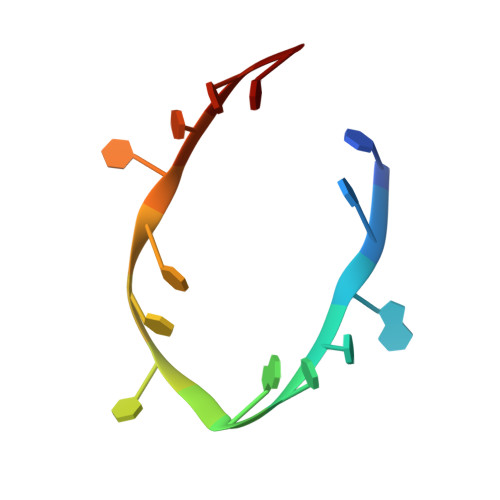
The Crystal Structure of the NHL Domain in Complex with RNA Reveals the Molecular Basis of Drosophila Brain-Tumor-Mediated Gene Regulation.
Loedige, I., Jakob, L., Treiber, T., Ray, D., Stotz, M., Treiber, N., Hennig, J., Cook, K.B., Morris, Q., Hughes, T.R., Engelmann, J.C., Krahn, M.P., Meister, G.(2015) Cell Rep 13: 1206-1220
- PubMed: 26527002
- DOI: https://doi.org/10.1016/j.celrep.2015.09.068
- Primary Citation of Related Structures:
4ZLR - PubMed Abstract:
TRIM-NHL proteins are conserved among metazoans and control cell fate decisions in various stem cell linages. The Drosophila TRIM-NHL protein Brain tumor (Brat) directs differentiation of neuronal stem cells by suppressing self-renewal factors. Brat is an RNA-binding protein and functions as a translational repressor. However, it is unknown which RNAs Brat regulates and how RNA-binding specificity is achieved. Using RNA immunoprecipitation and RNAcompete, we identify Brat-bound mRNAs in Drosophila embryos and define consensus binding motifs for Brat as well as a number of additional TRIM-NHL proteins, indicating that TRIM-NHL proteins are conserved, sequence-specific RNA-binding proteins. We demonstrate that Brat-mediated repression and direct RNA-binding depend on the identified motif and show that binding of the localization factor Miranda to the Brat-NHL domain inhibits Brat activity. Finally, to unravel the sequence specificity of the NHL domain, we crystallize the Brat-NHL domain in complex with RNA and present a high-resolution protein-RNA structure of this fold.
Organizational Affiliation:
Laboratory for RNA Biology, Biochemistry Center Regensburg, University of Regensburg, 93053 Regensburg, Germany. Electronic address: inga.loedige@embl.de.