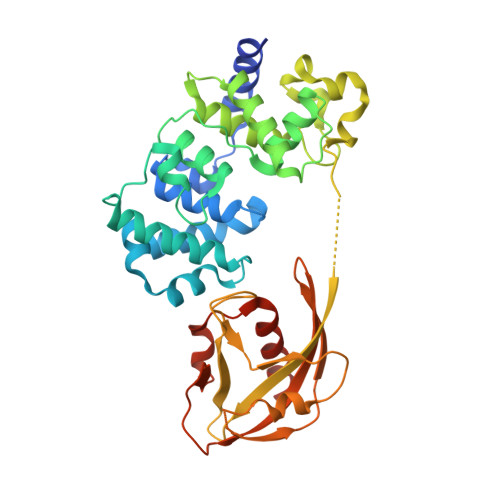
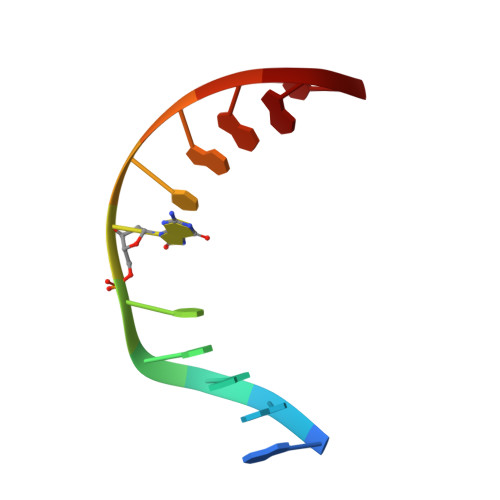
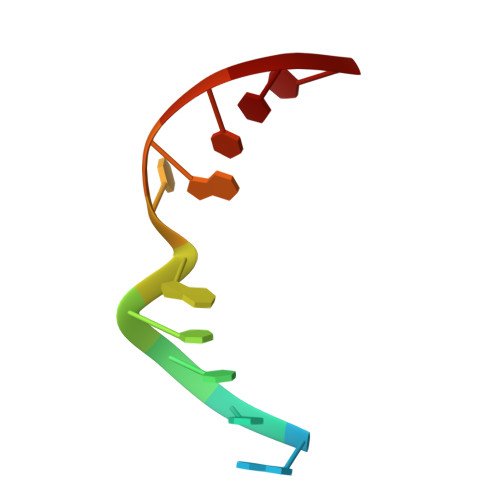
Structural Basis for Avoidance of Promutagenic DNA Repair by MutY Adenine DNA Glycosylase.
Wang, L., Lee, S.J., Verdine, G.L.(2015) J Biol Chem 290: 17096-17105
- PubMed: 25995449
- DOI: https://doi.org/10.1074/jbc.M115.657866
- Primary Citation of Related Structures:
4YOQ, 4YPH, 4YPR - PubMed Abstract:
The highly mutagenic A:oxoG (8-oxoguanine) base pair in DNA most frequently arises by aberrant replication of the primary oxidative lesion C:oxoG. This lesion is particularly insidious because neither of its constituent nucleobases faithfully transmit genetic information from the original C:G base pair. Repair of A:oxoG is initiated by adenine DNA glycosylase, which catalyzes hydrolytic cleavage of the aberrant A nucleobase from the DNA backbone. These enzymes, MutY in bacteria and MUTYH in humans, scrupulously avoid processing of C:oxoG because cleavage of the C residue in C:oxoG would actually promote mutagenic conversion to A:oxoG. Here we analyze the structural basis for rejection of C:oxoG by MutY, using a synthetic crystallography approach to capture the enzyme in the process of inspecting the C:oxoG anti-substrate, with which it ordinarily binds only fleetingly. We find that MutY uses two distinct strategies to avoid presentation of C to the enzyme active site. Firstly, MutY possesses an exo-site that serves as a decoy for C, and secondly, repulsive forces with a key active site residue prevent stable insertion of C into the nucleobase recognition pocket within the enzyme active site.
Organizational Affiliation:
From the Departments of Chemistry and Chemical Biology.