Structure of the carboxy-terminal domain of Mycobacterium tuberculosis CarD protein: an essential rRNA transcriptional regulator.
Gangwar, S.P., Meena, S.R., Saxena, A.K.(2014) Acta Crystallogr F Struct Biol Commun 70: 160-165
- PubMed: 24637748
- DOI: https://doi.org/10.1107/S2053230X13034407
- Primary Citation of Related Structures:
4KMC - PubMed Abstract:
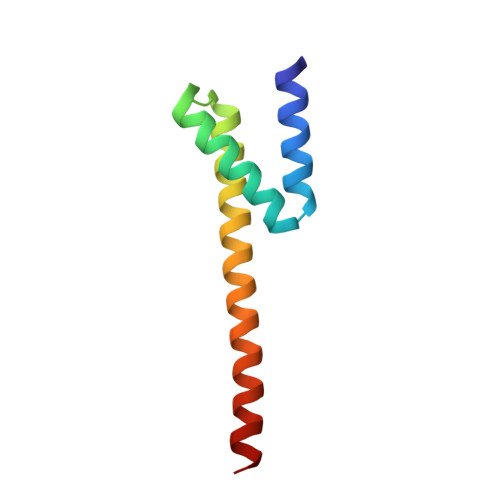
The CarD protein is highly expressed in mycobacterial strains under basal conditions and is transcriptionally induced during multiple types of genotoxic stress and starvation. The CarD protein binds the β subunit of RNA polymerase and influences gene expression. The disruption of interactions between CarD and the β subunit of RNA polymerase has a significant effect on mycobacterial survival, resistance to stress and pathogenesis. To understand the structure of CarD and its interaction with the β subunit of RNA polymerase, Mycobacterium tuberculosis CarD (MtbCarD) and the Thermus aquaticus RNA polymerase β subunit were recombinantly expressed and purified. Secondary-structure analysis using circular-dichroism spectroscopy indicated that MtbCarD contains ∼ 60% α-helix, ∼ 7% β-sheet and ∼ 33% random-coil structure. The C-terminal domain of MtbCarD (CarD(83-161)) was crystallized and its X-ray structure was determined at 2.1 Å resolution. CarD(83-161) forms a distorted Y-shaped structure containing bundles of three helices connected by a loop. The residues forming the distorted Y-shaped structure are highly conserved in CarD sequences from other mycobacterial species. Comparison of the CarD(83-161) structure with the recently determined full-length M. tuberculosis and T. thermophilus CarD crystal structures revealed structural differences in residues 141-161 of the C-terminal domain of the CarD(83-161) structure. The structural changes in the CarD(83-161) structure occurred owing to proteolysis and crystallization artifacts.
Organizational Affiliation:
Structural Biology Laboratory, School of Life Sciences, Jawaharlal Nehru University, New Delhi 110 067, India.