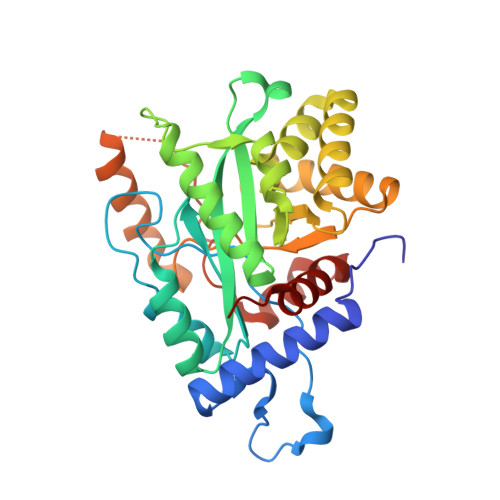
Proofreading exonuclease on a tether: the complex between the E. coli DNA polymerase III subunits alpha, {varepsilon}, theta and beta reveals a highly flexible arrangement of the proofreading domain
Ozawa, K., Horan, N.P., Robinson, A., Yagi, H., Hill, F.R., Jergic, S., Xu, Z.Q., Loscha, K.V., Li, N., Tehei, M., Oakley, A.J., Otting, G., Huber, T., Dixon, N.E.(2013) Nucleic Acids Res 41: 5354-5367
- PubMed: 23580545
- DOI: https://doi.org/10.1093/nar/gkt162
- Primary Citation of Related Structures:
4GX8, 4GX9 - PubMed Abstract:
A complex of the three (αεθ) core subunits and the β2 sliding clamp is responsible for DNA synthesis by Pol III, the Escherichia coli chromosomal DNA replicase. The 1.7 Å crystal structure of a complex between the PHP domain of α (polymerase) and the C-terminal segment of ε (proofreading exonuclease) subunits shows that ε is attached to α at a site far from the polymerase active site. Both α and ε contain clamp-binding motifs (CBMs) that interact simultaneously with β2 in the polymerization mode of DNA replication by Pol III. Strengthening of both CBMs enables isolation of stable αεθ:β2 complexes. Nuclear magnetic resonance experiments with reconstituted αεθ:β2 demonstrate retention of high mobility of a segment of 22 residues in the linker that connects the exonuclease domain of ε with its α-binding segment. In spite of this, small-angle X-ray scattering data show that the isolated complex with strengthened CBMs has a compact, but still flexible, structure. Photo-crosslinking with p-benzoyl-L-phenylalanine incorporated at different sites in the α-PHP domain confirm the conformational variability of the tether. Structural models of the αεθ:β2 replicase complex with primer-template DNA combine all available structural data.
Organizational Affiliation:
School of Chemistry, University of Wollongong, Northfields Avenue, Wollongong, NSW 2522, Australia.