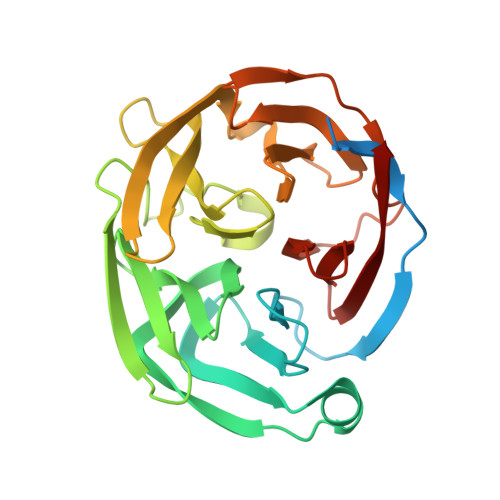
The Olfactomedin Domain from Gliomedin is a Beta-Propeller with Unique Structural Properties.
Han, H., Kursula, P.(2015) J Biol Chem 290: 3612
- PubMed: 25525261
- DOI: https://doi.org/10.1074/jbc.M114.627547
- Primary Citation of Related Structures:
4D77, 4D7C - PubMed Abstract:
All members of the olfactomedin (OLF) family have a conserved extracellular OLF domain, for which a structure has not been available. We present here the crystal structure of the OLF domain from gliomedin. Gliomedin is a protein expressed by Schwann cells in peripheral nerves, important for the formation of the nodes of Ranvier. Gliomedin interacts with neuronal cell adhesion molecules, such as neurofascin, but the structural details of the interaction are not known. The structure of the OLF domain presents a five-bladed β-propeller fold with unusual geometric properties. The symmetry of the structure is not 5-fold, but rather reveals a twisted arrangement. The conserved top face of the gliomedin OLF domain is likely to be important for binding to neuronal ligands. Our results provide a structural basis for the functions of gliomedin in Schwann cells, enable the understanding of the role of the gliomedin OLF domain in autoimmune neuropathies, and unravel the locations of human disease-causing mutations in other OLF family members, including myocilin.
Organizational Affiliation:
From the Faculty of Biochemistry and Molecular Medicine and Biocenter Oulu, University of Oulu, 90014 Oulu, Finland, the German Electron Synchrotron (DESY), 22607 Hamburg, Germany, and.