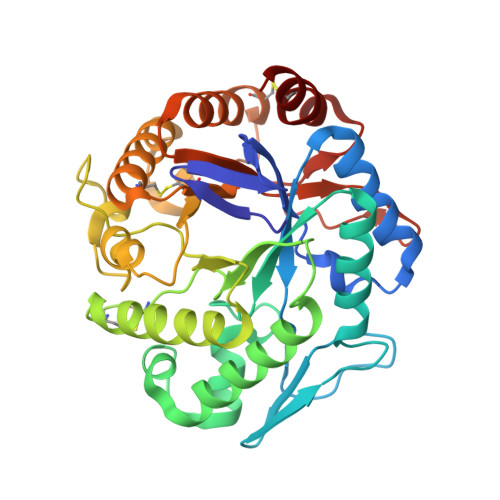
Purification, cloning, functional expression, structure, and characterization of a thermostable beta-mannanase from Talaromyces trachyspermus and its efficiency in production of mannooligosaccharides from coffee wastes
Ichinose, H., Suzuki, K., Yuki, M., Michikawa, M., Sato, H., Kamino, K., Ogasawara, W., Fushinobu, S., Kaneko, S.To be published.