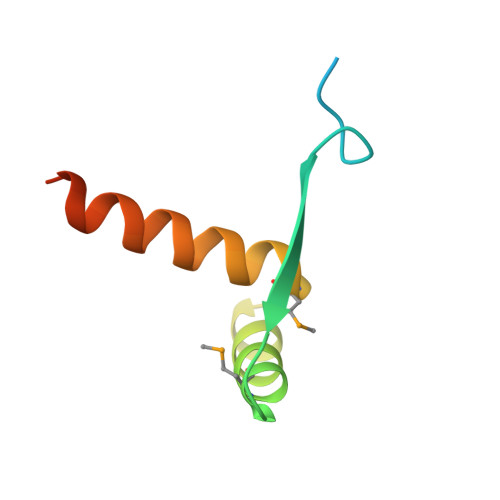
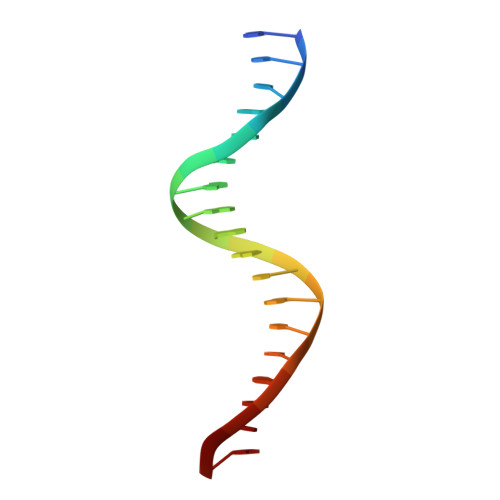
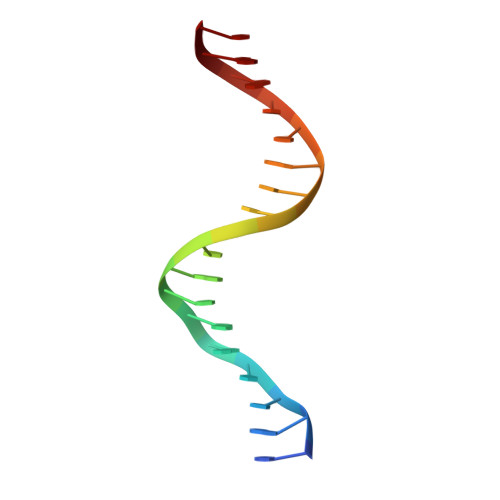
The Transcription Factor AmrZ Utilizes Multiple DNA Binding Modes to Recognize Activator and Repressor Sequences of Pseudomonas aeruginosa Virulence Genes.
Pryor Jr., E.E., Waligora, E.A., Xu, B., Dellos-Nolan, S., Wozniak, D.J., Hollis, T.(2012) PLoS Pathog 8: e1002648-e1002648
- PubMed: 22511872
- DOI: https://doi.org/10.1371/journal.ppat.1002648
- Primary Citation of Related Structures:
3QOQ - PubMed Abstract:
AmrZ, a member of the Ribbon-Helix-Helix family of DNA binding proteins, functions as both a transcriptional activator and repressor of multiple genes encoding Pseudomonas aeruginosa virulence factors. The expression of these virulence factors leads to chronic and sustained infections associated with worsening prognosis. In this study, we present the X-ray crystal structure of AmrZ in complex with DNA containing the repressor site, amrZ1. Binding of AmrZ to this site leads to auto-repression. AmrZ binds this DNA sequence as a dimer-of-dimers, and makes specific base contacts to two half sites, separated by a five base pair linker region. Analysis of the linker region shows a narrowing of the minor groove, causing significant distortions. AmrZ binding assays utilizing sequences containing variations in this linker region reveals that secondary structure of the DNA, conferred by the sequence of this region, is an important determinant in binding affinity. The results from these experiments allow for the creation of a model where both intrinsic structure of the DNA and specific nucleotide recognition are absolutely necessary for binding of the protein. We also examined AmrZ binding to the algD promoter, which results in activation of the alginate exopolysaccharide biosynthetic operon, and found the protein utilizes different interactions with this site. Finally, we tested the in vivo effects of this differential binding by switching the AmrZ binding site at algD, where it acts as an activator, for a repressor binding sequence and show that differences in binding alone do not affect transcriptional regulation.
Organizational Affiliation:
Department of Biochemistry and Center for Structural Biology, Wake Forest School of Medicine, Winston-Salem, North Carolina, United States of America.