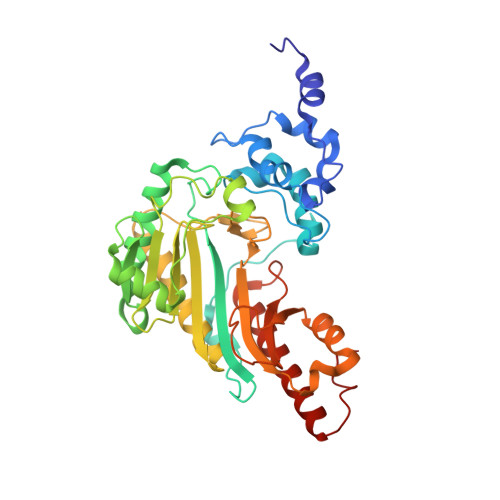
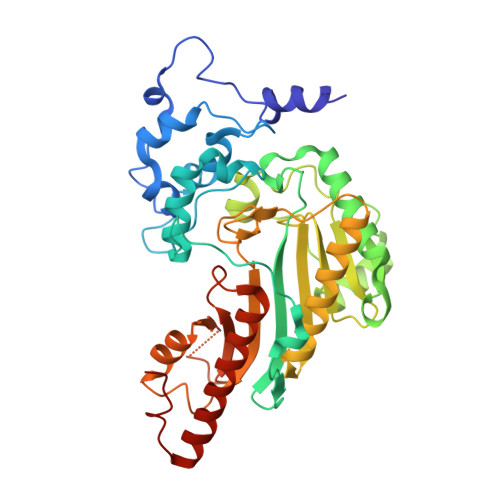
Structural basis for the mechanism and substrate specificity of glycocyamine kinase, a phosphagen kinase family member.
Lim, K., Pullalarevu, S., Surabian, K.T., Howard, A., Suzuki, T., Moult, J., Herzberg, O.(2010) Biochemistry 49: 2031-2041
- PubMed: 20121101
- DOI: https://doi.org/10.1021/bi9020988
- Primary Citation of Related Structures:
3L2D, 3L2E, 4V7N - PubMed Abstract:
Glycocyamine kinase (GK), a member of the phosphagen kinase family, catalyzes the Mg(2+)-dependent reversible phosphoryl group transfer of the N-phosphoryl group of phosphoglycocyamine to ADP to yield glycocyamine and ATP. This reaction helps to maintain the energy homeostasis of the cell in some multicelullar organisms that encounter high and variable energy turnover. GK from the marine worm Namalycastis sp. is heterodimeric, with two homologous polypeptide chains, alpha and beta, derived from a common pre-mRNA by mutually exclusive N-terminal alternative exons. The N-terminal exon of GKbeta encodes a peptide that is different in sequence and is 16 amino acids longer than that encoded by the N-terminal exon of GKalpha. The crystal structures of recombinant GKalphabeta and GKbetabeta from Namalycastis sp. were determined at 2.6 and 2.4 A resolution, respectively. In addition, the structure of the GKbetabeta was determined at 2.3 A resolution in complex with a transition state analogue, Mg(2+)-ADP-NO(3)(-)-glycocyamine. Consistent with the sequence homology, the GK subunits adopt the same overall fold as that of other phosphagen kinases of known structure (the homodimeric creatine kinase (CK) and the monomeric arginine kinase (AK)). As with CK, the GK N-termini mediate the dimer interface. In both heterodimeric and homodimeric GK forms, the conformations of the two N-termini are asymmetric, and the asymmetry is different than that reported previously for the homodimeric CKs from several organisms. The entire polypeptide chains of GKalphabeta are structurally defined, and the longer N-terminus of the beta subunit is anchored at the dimer interface. In GKbetabeta the 24 N-terminal residues of one subunit and 11 N-terminal residues of the second subunit are disordered. This observation is consistent with a proposal that the GKalphabeta amino acids involved in the interface formation were optimized once a heterodimer emerged as the physiological form of the enzyme. As a consequence, the homodimer interface (either solely alpha or solely beta chains) has been corrupted. In the unbound state, GK exhibits an open conformation analogous to that observed with ligand-free CK or AK. Upon binding the transition state analogue, both subunits of GK undergo the same closure motion that clasps the transition state analogue, in contrast to the transition state analogue complexes of CK, where the corresponding transition state analogue occupies only one subunit, which undergoes domain closure. The active site environments of the GK, CK, and AK at the bound states reveal the structural determinants of substrate specificity. Despite the equivalent binding in both active sites of the GK dimer, the conformational asymmetry of the N-termini is retained. Thus, the coupling between the structural asymmetry and negative cooperativity previously proposed for CK is not supported in the case of GK.
Organizational Affiliation:
Center for Advanced Research in Biotechnology, University of Maryland Biotechnology Institute, Rockville, Maryland 20850, USA.