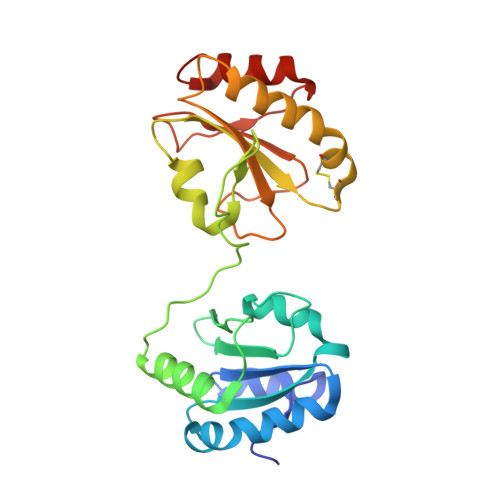
Redox-dependent conformational transition of catalytic domain of protein disulfide isomerase indicated by crystal structure-based molecular dynamics simulation
Inagaki, K., Satoh, T., Itoh, S.G., Okumura, H., Kato, K.(2015) Chem Phys Lett 618: 203-207