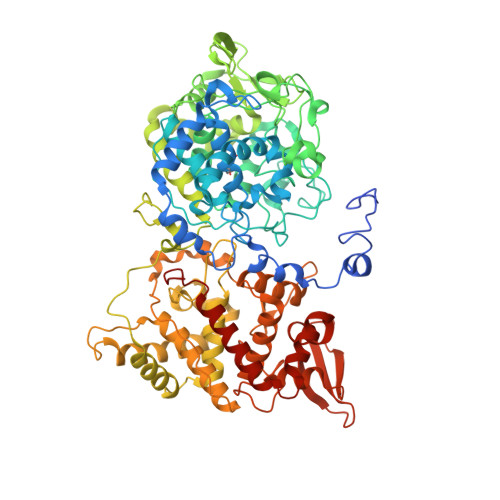
High Conformational Stability of Secreted Eukaryotic Catalase-peroxidases: ANSWERS FROM FIRST CRYSTAL STRUCTURE AND UNFOLDING STUDIES.
Zamocky, M., Garcia-Fernandez, Q., Gasselhuber, B., Jakopitsch, C., Furtmuller, P.G., Loewen, P.C., Fita, I., Obinger, C., Carpena, X.(2012) J Biol Chem 287: 32254-32262
- PubMed: 22822072
- DOI: https://doi.org/10.1074/jbc.M112.384271
- Primary Citation of Related Structures:
3UT2 - PubMed Abstract:
Catalase-peroxidases (KatGs) are bifunctional heme enzymes widely spread in archaea, bacteria, and lower eukaryotes. Here we present the first crystal structure (1.55 Å resolution) of an eukaryotic KatG, the extracellular or secreted enzyme from the phytopathogenic fungus Magnaporthe grisea. The heme cavity of the homodimeric enzyme is similar to prokaryotic KatGs including the unique distal (+)Met-Tyr-Trp adduct (where the Trp is further modified by peroxidation) and its associated mobile arginine. The structure also revealed several conspicuous peculiarities that are fully conserved in all secreted eukaryotic KatGs. Peculiarities include the wrapping at the dimer interface of the N-terminal elongations from the two subunits and cysteine residues that cross-link the two subunits. Differential scanning calorimetry and temperature- and urea-mediated unfolding followed by UV-visible, circular dichroism, and fluorescence spectroscopy combined with site-directed mutagenesis demonstrated that secreted eukaryotic KatGs have a significantly higher conformational stability as well as a different unfolding pattern when compared with intracellular eukaryotic and prokaryotic catalase-peroxidases. We discuss these properties with respect to the structure as well as the postulated roles of this metalloenzyme in host-pathogen interactions.
Organizational Affiliation:
Department of Chemistry, Division of Biochemistry, BOKU, University of Natural Resources and Life Sciences, A-1190 Vienna, Austria.