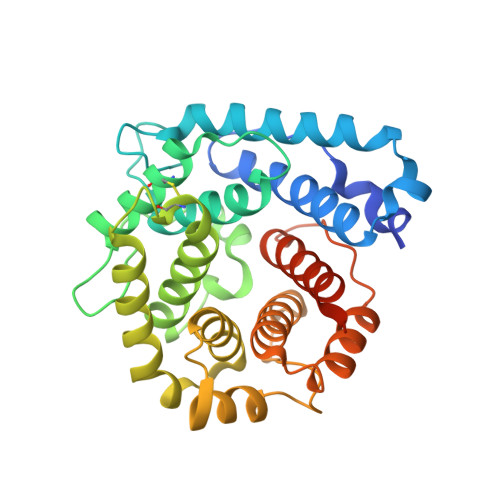
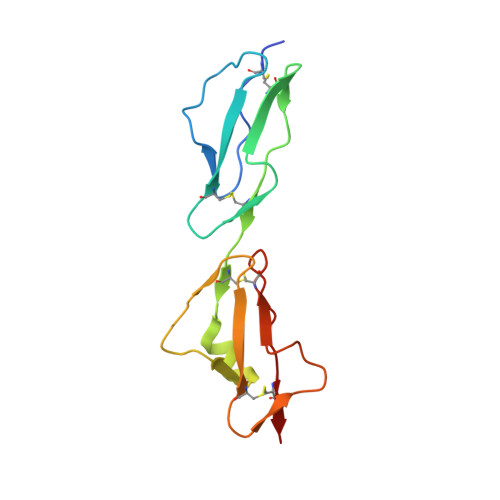
Structural and functional characterization of the product of disease-related factor h gene conversion.
Herbert, A.P., Kavanagh, D., Johansson, C., Morgan, H.P., Blaum, B.S., Hannan, J.P., Barlow, P.N., Uhrin, D.(2012) Biochemistry 51: 1874-1884
- PubMed: 22320225
- DOI: https://doi.org/10.1021/bi201689j
- Primary Citation of Related Structures:
3RJ3 - PubMed Abstract:
Numerous complement factor H (FH) mutations predispose patients to atypical hemolytic uremic syndrome (aHUS) and other disorders arising from inadequately regulated complement activation. No unifying structural or mechanistic consequences have been ascribed to these mutants beyond impaired self-cell protection. The S1191L and V1197A mutations toward the C-terminus of FH, which occur in patients singly or together, arose from gene conversion between CFH encoding FH and CFHR1 encoding FH-related 1. We show that neither single nor double mutations structurally perturbed recombinant proteins consisting of the FH C-terminal modules, 19 and 20 (FH19-20), although all three FH19-20 mutants were poor, compared to wild-type FH19-20, at promoting hemolysis of C3b-coated erythrocytes through competition with full-length FH. Indeed, our new crystal structure of the S1191L mutant of FH19-20 complexed with an activation-specific complement fragment, C3d, was nearly identical to that of the wild-type FH19-20:C3d complex, consistent with mutants binding to C3b with wild-type-like affinity. The S1191L mutation enhanced thermal stability of module 20, whereas the V1197A mutation dramatically decreased it. Thus, although mutant proteins were folded at 37 °C, they differ in conformational rigidity. Neither single substitutions nor double substitutions increased measurably the extent of FH19-20 self-association, nor did these mutations significantly affect the affinity of FH19-20 for three glycosaminoglycans, despite critical roles of module 20 in recognizing polyanionic self-surface markers. Unexpectedly, FH19-20 mutants containing Leu1191 self-associated on a heparin-coated surface to a higher degree than on surfaces coated with dermatan or chondroitin sulfates. Thus, potentially disease-related functional distinctions between mutants, and between FH and FH-related 1, may manifest in the presence of specific glycosaminoglycans.
Organizational Affiliation:
Edinburgh Biomolecular NMR Unit, EastChem School of Chemistry, University of Edinburgh, Edinburgh EH9 3JJ, UK.