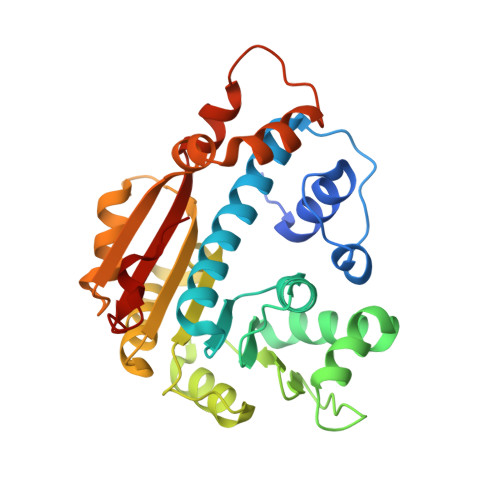
The structure of human leucine carboxyl methyltransferase 1 that regulates protein phosphatase PP2A
Tsai, M.L., Cronin, N., Djordjevic, S.(2011) Acta Crystallogr D Biol Crystallogr 67: 14-24
- PubMed: 21206058
- DOI: https://doi.org/10.1107/S0907444910042204
- Primary Citation of Related Structures:
3O7W - PubMed Abstract:
Leucine carboxyl methyltransferase 1 (LCMT1) methylates the terminal carboxyl group of the leucine 309 residue of human protein phosphatase 2A (PP2A). PP2A, a key regulator of many cellular processes, has recently generated additional interest as a potential cancer-therapeutic target. The status of PP2A methylation impacts upon the selection of the regulatory subunit by the PP2A core enzyme, thus directing its activity and subcellular localization. An X-ray crystal structure of human LCMT1 protein in complex with the cofactor S-adenosylmethionine (AdoMet) has been solved to a resolution of 2 Å. The structure enables the postulation of a mode of interaction with protein phosphatase PP2A and provides a platform for further functional studies of the regulation of methylation of PP2A.
Organizational Affiliation:
Institute of Structural and Molecular Biology, University College London, England.