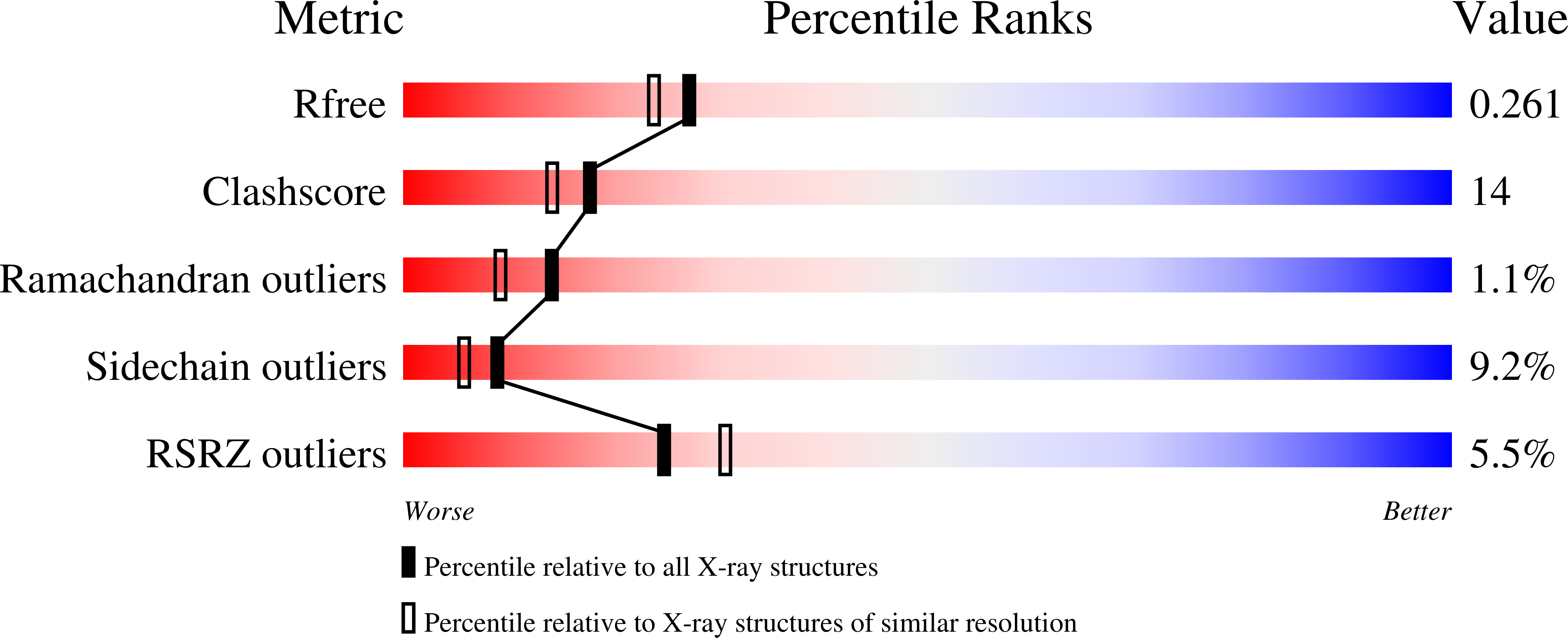
Structure and flexibility in cold-adapted iron superoxide dismutases: the case of the enzyme isolated from Pseudoalteromonas haloplanktis.
Merlino, A., Russo Krauss, I., Castellano, I., De Vendittis, E., Rossi, B., Conte, M., Vergara, A., Sica, F.(2010) J Struct Biol 172: 343-352
- PubMed: 20732427
- DOI: https://doi.org/10.1016/j.jsb.2010.08.008
- Primary Citation of Related Structures:
3LIO, 3LJ9, 3LJF - PubMed Abstract:
Superoxide dismutases (SODs) are metalloenzymes catalysing the dismutation of superoxide anion radicals into molecular oxygen and hydrogen peroxide. Here, we present the crystal structure of a cold-adapted Fe-SOD from the Antarctic eubacterium Pseudoalteromonas haloplanktis (PhSOD), and that of its complex with sodium azide. The structures were compared with those of the corresponding homologues having a high sequence identity with PhSOD, such as the mesophilic SOD from Escherichia coli (EcSOD) or Pseudomonas ovalis, and the psychrophilic SOD from Aliivibrio salmonicida (AsSOD). These enzymes shared a large structural similarity, such as a conserved tertiary structure and arrangement of the two monomers, an almost identical total number of inter- and intramolecular hydrogen bonds and salt bridges. However, the two cold-adapted SODs showed an increased flexibility of the active site residues with respect to their mesophilic homologues. Structural information was combined with a characterisation of the chemical and thermal stability performed by CD and fluorescence measurements. Despite of its psychrophilic origin, the denaturation temperature of PhSOD was comparable with that of the mesophilic EcSOD, whereas AsSOD showed a lower denaturation temperature. On the contrary, the values of the denaturant concentration at the transition midpoint were in line with the psychrophilic/mesophilic origin of the proteins. These data provide additional support to the hypothesis that cold-adapted enzymes achieve efficient catalysis at low temperature, by increasing the flexibility of their active site; moreover, our results underline how fine structural modifications can alter enzyme flexibility and/or stability without compromising the overall structure of typical rigid enzymes, such as SODs.
Organizational Affiliation:
Dipartimento di Chimica, Università di Napoli Federico II, Complesso Universitario Monte S. Angelo, Via Cinthia, I-80126 Naples, Italy.