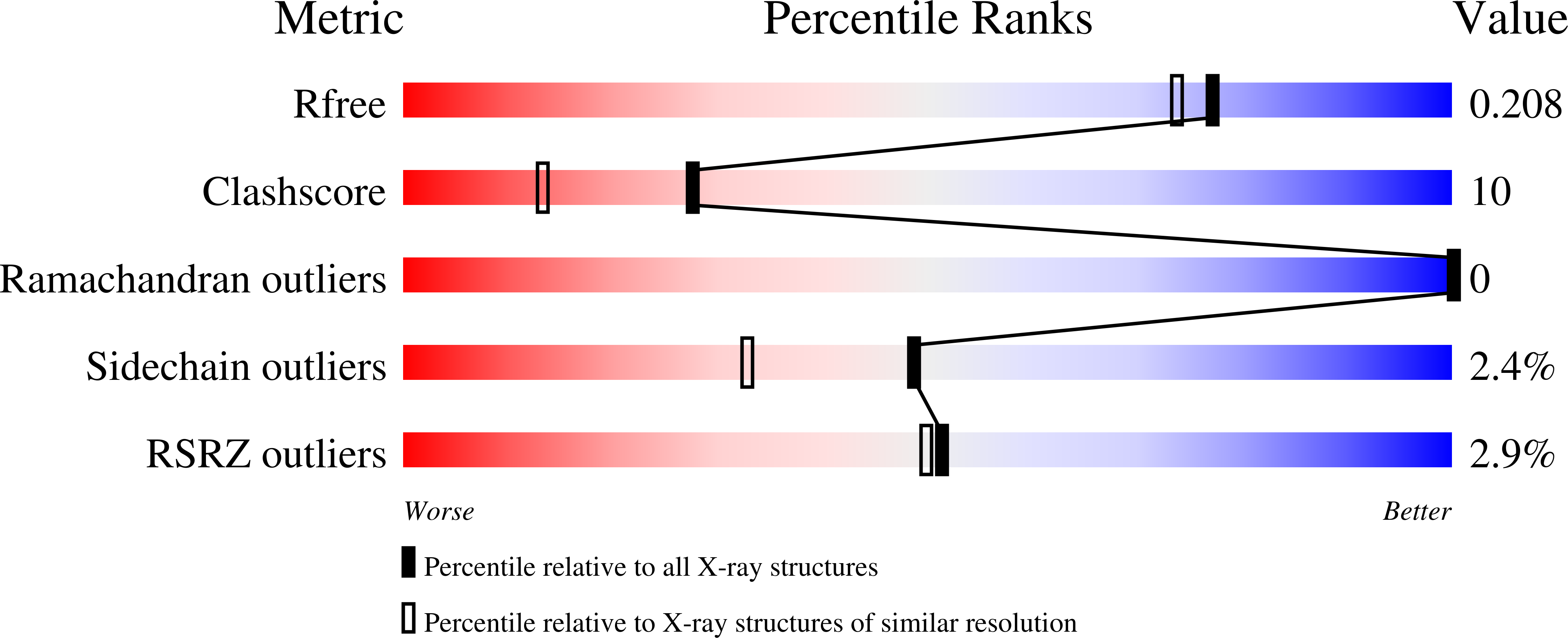
Fragment screening of inhibitors for MIF tautomerase reveals a cryptic surface binding site.
McLean, L.R., Zhang, Y., Li, H., Choi, Y.M., Han, Z., Vaz, R.J., Li, Y.(2010) Bioorg Med Chem Lett 20: 1821-1824
- PubMed: 20185308
- DOI: https://doi.org/10.1016/j.bmcl.2010.02.009
- Primary Citation of Related Structures:
3L5P, 3L5R, 3L5S, 3L5T, 3L5U, 3L5V - PubMed Abstract:
In the course of a fragment screening campaign by in silico docking followed by X-ray crystallography, a novel binding site for migration inhibitory factor (MIF) inhibitors was demonstrated. The site is formed by rotation of the side-chain of Tyr-36 to reveal a surface binding site in MIF that is hydrophobic and surrounded by aromatic side-chain residues. The crystal structures of two small inhibitors that bind to this site and of a quinolinone inhibitor, that spans the canonical deep pocket near Pro-1 and the new surface binding site, have been solved. These results suggest new opportunities for structure-based design of MIF inhibitors.
Organizational Affiliation:
Discovery Research, Sanofi-aventis, Bridgewater, NJ 08807, USA. Larry.Mclean@sanofi-aventis.com