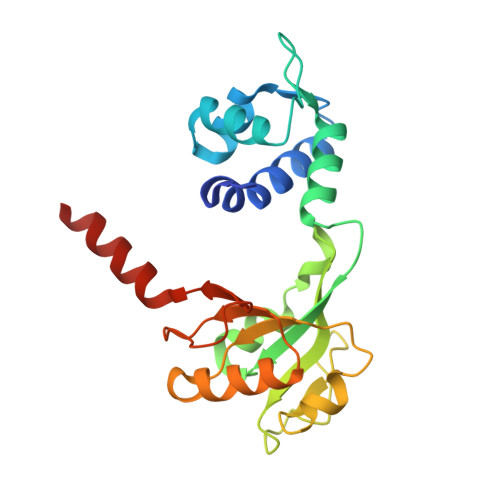
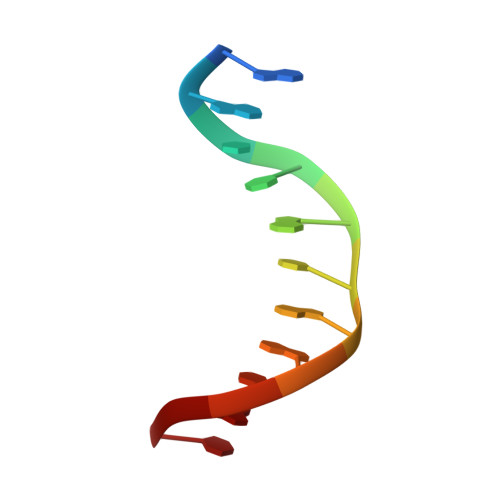
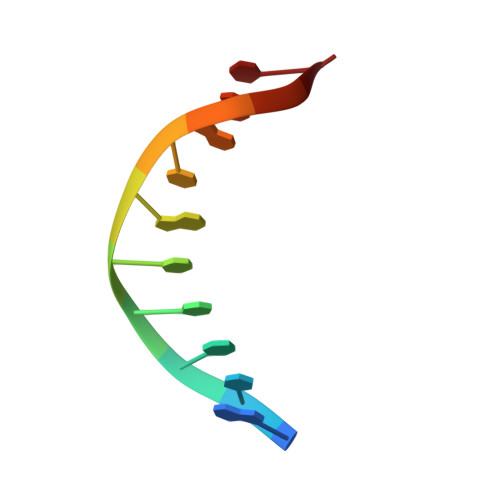
NAD+ pool depletion as a signal for the Rex regulon involved in Streptococcus agalactiae virulence.
Franza, T., Rogstam, A., Thiyagarajan, S., Sullivan, M.J., Derre-Bobillot, A., Bauer, M.C., Goh, K.G.K., Da Cunha, V., Glaser, P., Logan, D.T., Ulett, G.C., von Wachenfeldt, C., Gaudu, P.(2021) PLoS Pathog 17: e1009791-e1009791
- PubMed: 34370789
- DOI: https://doi.org/10.1371/journal.ppat.1009791
- Primary Citation of Related Structures:
3KEO, 3KEQ, 3KET - PubMed Abstract:
In many Gram-positive bacteria, the redox-sensing transcriptional repressor Rex controls central carbon and energy metabolism by sensing the intra cellular balance between the reduced and oxidized forms of nicotinamide adenine dinucleotide; the NADH/NAD+ ratio. Here, we report high-resolution crystal structures and characterization of a Rex ortholog (Gbs1167) in the opportunistic pathogen, Streptococcus agalactiae, also known as group B streptococcus (GBS). We present structures of Rex bound to NAD+ and to a DNA operator which are the first structures of a Rex-family member from a pathogenic bacterium. The structures reveal the molecular basis of DNA binding and the conformation alterations between the free NAD+ complex and DNA-bound form of Rex. Transcriptomic analysis revealed that GBS Rex controls not only central metabolism, but also expression of the monocistronic rex gene as well as virulence gene expression. Rex enhances GBS virulence after disseminated infection in mice. Mechanistically, NAD+ stabilizes Rex as a repressor in the absence of NADH. However, GBS Rex is unique compared to Rex regulators previously characterized because of its sensing mechanism: we show that it primarily responds to NAD+ levels (or growth rate) rather than to the NADH/NAD+ ratio. These results indicate that Rex plays a key role in GBS pathogenicity by modulating virulence factor gene expression and carbon metabolism to harvest nutrients from the host.
Organizational Affiliation:
Micalis Institute, INRAE, AgroParisTech, Université Paris-Saclay, Jouy-en-Josas, France.