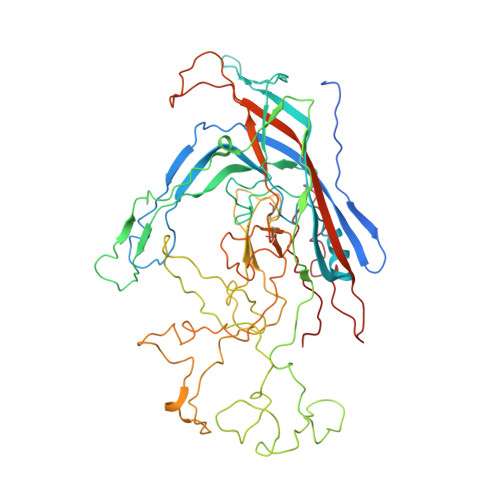
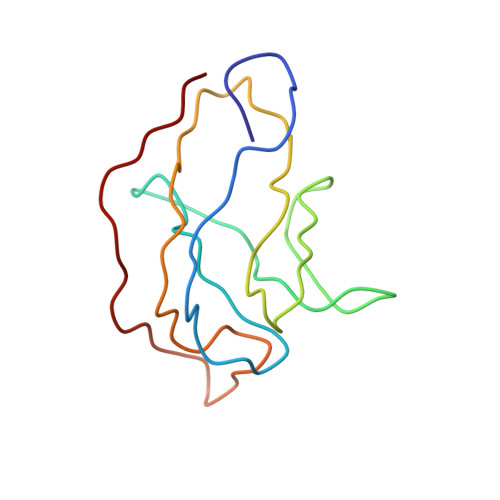
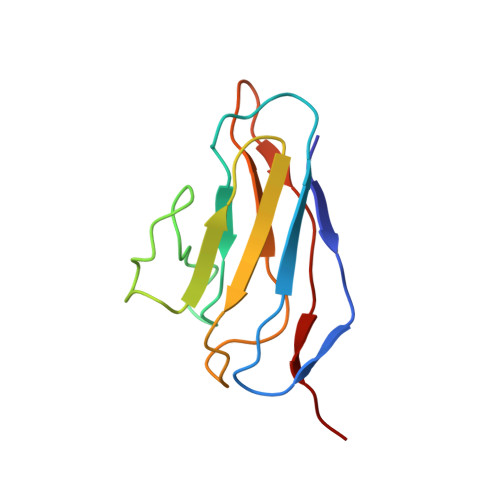
Near-Atomic Resolution Structure of a Highly Neutralizing Fab Bound to Canine Parvovirus.
Organtini, L.J., Lee, H., Iketani, S., Huang, K., Ashley, R.E., Makhov, A.M., Conway, J.F., Parrish, C.R., Hafenstein, S.(2016) J Virol 90: 9733-9742
- PubMed: 27535057
- DOI: https://doi.org/10.1128/JVI.01112-16
- Primary Citation of Related Structures:
3JCX - PubMed Abstract:
Canine parvovirus (CPV) is a highly contagious pathogen that causes severe disease in dogs and wildlife. Previously, a panel of neutralizing monoclonal antibodies (MAb) raised against CPV was characterized. An antibody fragment (Fab) of MAb E was found to neutralize the virus at low molar ratios. Using recent advances in cryo-electron microscopy (cryo-EM), we determined the structure of CPV in complex with Fab E to 4.1 Å resolution, which allowed de novo building of the Fab structure. The footprint identified was significantly different from the footprint obtained previously from models fitted into lower-resolution maps. Using single-chain variable fragments, we tested antibody residues that control capsid binding. The near-atomic structure also revealed that Fab binding had caused capsid destabilization in regions containing key residues conferring receptor binding and tropism, which suggests a mechanism for efficient virus neutralization by antibody. Furthermore, a general technical approach to solving the structures of small molecules is demonstrated, as binding the Fab to the capsid allowed us to determine the 50-kDa Fab structure by cryo-EM.
Organizational Affiliation:
Department of Medicine, The Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA.