Crystal structure of putative cystathionine beta-lyase involved in aluminum resistance (NP_348457.1) from Clostridium acetobutylicum at 2.00 A resolution
Joint Center for Structural Genomics (JCSG)To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cystathionine beta-lyase family protein, YNBB B.subtilis ortholog | 427 | Clostridium acetobutylicum | Mutation(s): 0 Gene Names: CA_C1833, NP_348457.1 | 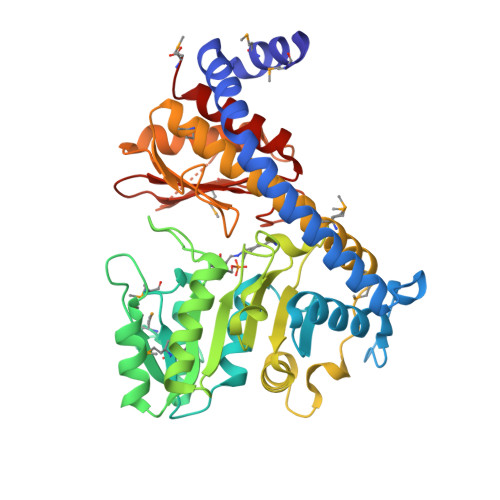 | |
UniProt | |||||
Find proteins for Q97I22 (Clostridium acetobutylicum (strain ATCC 824 / DSM 792 / JCM 1419 / LMG 5710 / VKM B-1787)) Explore Q97I22 Go to UniProtKB: Q97I22 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q97I22 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| EDO Query on EDO | F [auth A] G [auth A] H [auth A] I [auth A] J [auth A] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| NA Query on NA | E [auth A], K [auth B], Q [auth C], X [auth D] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Modified Residues 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| LLP Query on LLP | A, B, C, D | L-PEPTIDE LINKING | C14 H22 N3 O7 P |  | LYS |
| MSE Query on MSE | A, B, C, D | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 58.83 | α = 90 |
| b = 186.62 | β = 103.06 |
| c = 93.71 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| PHENIX | refinement |
| SHELX | phasing |
| MolProbity | model building |
| XSCALE | data scaling |
| PDB_EXTRACT | data extraction |
| XDS | data reduction |
| SHELXD | phasing |
| autoSHARP | phasing |