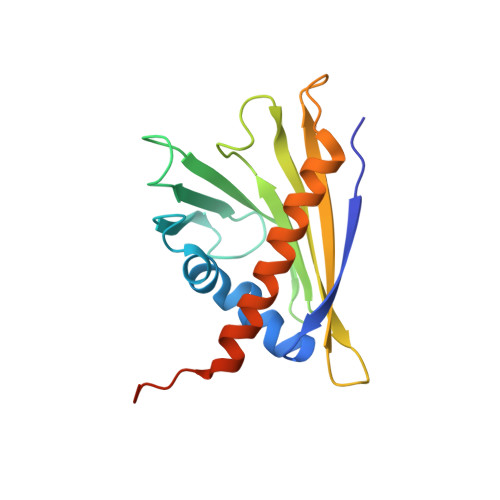
Solution structure of the strawberry allergen Fra a 1.
Seutter von Loetzen, C., Schweimer, K., Schwab, W., Rosch, P., Hartl-Spiegelhauer, O.(2012) Biosci Rep 32: 567-575
- PubMed: 22913709
- DOI: https://doi.org/10.1042/BSR20120058
- Primary Citation of Related Structures:
2LPX - PubMed Abstract:
The PR10 family protein Fra a 1E from strawberry (Fragaria x ananassa) is down-regulated in white strawberry mutants, and transient RNAi (RNA interference)-mediated silencing experiments confirmed that Fra a 1 is involved in fruit pigment synthesis. In the present study, we determined the solution structure of Fra a 1E. The protein fold is identical with that of other members of the PR10 protein family and consists of a seven-stranded antiparallel β-sheet, two short V-shaped α-helices and a long C-terminal α-helix that encompass a hydrophobic pocket. Whereas Fra a 1E contains the glycine-rich loop that is highly conserved throughout the protein family, the volume of the hydrophobic pocket and the size of its entrance are much larger than expected. The three-dimensional structure may shed some light on its physiological function and may help to further understand the role of PR10 proteins in plants.
Organizational Affiliation:
Lehrstuhl Biopolymere und Forschungszentrum für Bio-Makromoleküle, Universität Bayreuth, Bayreuth, Germany.