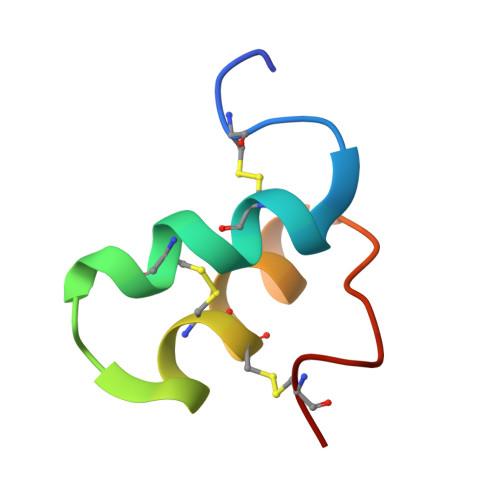
A helical conotoxin from Conus imperialis has a novel cysteine framework and defines a new superfamily.
Ye, M., Khoo, K.K., Xu, S., Zhou, M., Boonyalai, N., Perugini, M.A., Shao, X., Chi, C., Galea, C.A., Wang, C., Norton, R.S.(2012) J Biol Chem 287: 14973-14983
- PubMed: 22399292
- DOI: https://doi.org/10.1074/jbc.M111.334615
- Primary Citation of Related Structures:
2LMZ - PubMed Abstract:
Cone snail venoms are a rich source of peptides, many of which are potent and selective modulators of ion channels and receptors. Here we report the isolation and characterization of two novel conotoxins from the venom of Conus imperialis. These two toxins contain a novel cysteine framework, C-C-C-CC-C, which has not been found in other conotoxins described to date. We name it framework XXIII and designate the two toxins im23a and im23b; cDNAs of these toxins exhibit a novel signal peptide sequence, which defines a new K-superfamily. The disulfide connectivity of im23a has been mapped by chemical mapping of partially reduced intermediates and by NMR structure calculations, both of which establish a I-II, III-IV, V-VI pattern of disulfide bridges. This pattern was also confirmed by synthesis of im23a with orthogonal protection of individual cysteine residues. The solution structure of im23a reveals that im23a adopts a novel helical hairpin fold. A cluster of acidic residues on the surface of the molecule is able to bind calcium. The biological activity of the native and recombinant peptides was tested by injection into mice intracranially and intravenously to assess the effects on the central and peripheral nervous systems, respectively. Intracranial injection of im23a or im23b into mice induced excitatory symptoms; however, the biological target of these new toxins has yet to be identified.
Organizational Affiliation:
Institute of Protein Research, Tongji University, 1239 Siping Road, Shanghai 20092, China.