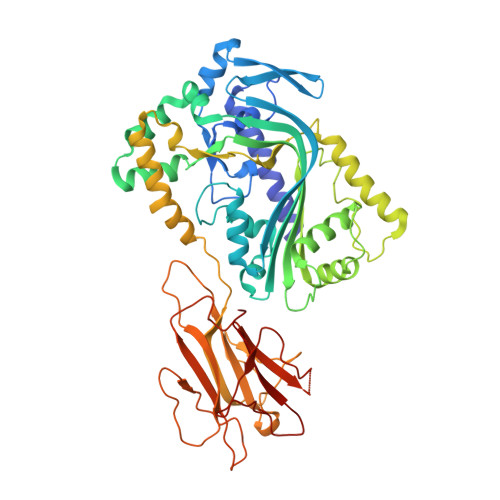
A common fold mediates vertebrate defense and bacterial attack
Rosado, C.J., Buckle, A.M., Law, R.H., Butcher, R.E., Kan, W.T., Bird, C.H., Ung, K., Browne, K.A., Baran, K., Bashtannyk-Puhalovich, T.A., Faux, N.G., Wong, W., Porter, C.J., Pike, R.N., Ellisdon, A.M., Pearce, M.C., Bottomley, S.P., Emsley, J., Smith, A.I., Rossjohn, J., Hartland, E.L., Voskoboinik, I., Trapani, J.A., Bird, P.I., Dunstone, M.A., Whisstock, J.C.(2007) Science 317: 1548-1551
- PubMed: 17717151
- DOI: https://doi.org/10.1126/science.1144706
- Primary Citation of Related Structures:
2QP2 - PubMed Abstract:
Proteins containing membrane attack complex/perforin (MACPF) domains play important roles in vertebrate immunity, embryonic development, and neural-cell migration. In vertebrates, the ninth component of complement and perforin form oligomeric pores that lyse bacteria and kill virus-infected cells, respectively. However, the mechanism of MACPF function is unknown. We determined the crystal structure of a bacterial MACPF protein, Plu-MACPF from Photorhabdus luminescens, to 2.0 angstrom resolution. The MACPF domain reveals structural similarity with poreforming cholesterol-dependent cytolysins (CDCs) from Gram-positive bacteria. This suggests that lytic MACPF proteins may use a CDC-like mechanism to form pores and disrupt cell membranes. Sequence similarity between bacterial and vertebrate MACPF domains suggests that the fold of the CDCs, a family of proteins important for bacterial pathogenesis, is probably used by vertebrates for defense against infection.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Monash University, Clayton, VIC 3800, Australia.