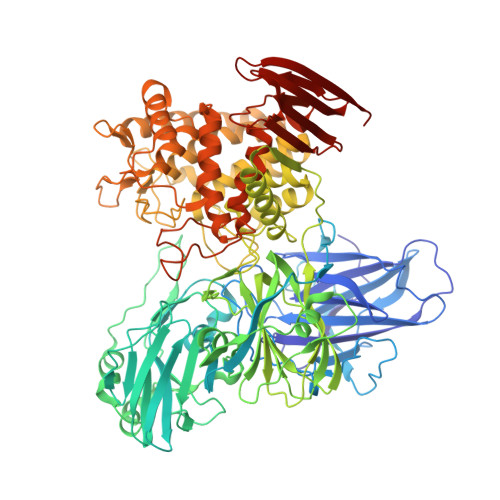
Crystal Structure of Glycoside Hydrolase Family 78 alpha-L-Rhamnosidase from Bacillus sp. GL1
Cui, Z., Maruyama, Y., Mikami, B., Hashimoto, W., Murata, K.(2007) J Mol Biol 374: 384-398
- PubMed: 17936784
- DOI: https://doi.org/10.1016/j.jmb.2007.09.003
- Primary Citation of Related Structures:
2OKX - PubMed Abstract:
alpha-L-Rhamnosidase (EC 3.2.1.40) catalyzes the hydrolytic release of rhamnose from polysaccharides and glycosides. Bacillus sp. GL1 alpha-L-rhamnosidase (RhaB), a member of glycoside hydrolase (GH) family 78, is responsible for degrading the bacterial biofilm gellan, and also functions as a debittering agent for citrus fruit in the food and beverage industries through the release of rhamnose from plant glycoside, naringin. The X-ray crystal structure of RhaB was determined by single-wavelength anomalous diffraction using a selenomethionine derivative and refined at 1.9 A resolution with a final R-factor of 18.2%. As is seen in the homodimeric form of the active enzyme, the structure of RhaB in crystal packing is a homodimer containing 1908 amino acids (residues 3-956), 43 glycerol molecules, four calcium ions, and 1755 water molecules. The overall structure consists of five domains, four of which are beta-sandwich structures designated as domains N, D1, D2, and C, and an (alpha/alpha)(6)-barrel structure designated as domain A. Structural comparison by DALI showed that RhaB shares its highest level of structural similarity with chitobiose phosphorylase (Z score of 25.3). The structure of RhaB in complex with the reaction product rhamnose (inhibitor constant, K(i)=1.8 mM) was also determined and refined at 2.1 A with a final R-factor of 19.5%. Rhamnose is bound to the deep cleft of the (alpha/alpha)(6)-barrel domain, as is seen in the clan-L GHs. Several negatively charged residues, such as Asp567, Glu572, Asp579, and Glu841, conserved in GH family 78 enzymes, interact with rhamnose, and RhaB mutants of these residues have drastically reduced enzyme activity, indicating that the residues are crucial for enzyme catalysis and/or substrate binding. To our knowledge, this is the first report on the determination of the crystal structure of alpha-L-rhamnosidase and identification of its clan-L (alpha/alpha)(6)-barrel as a catalytic domain.
Organizational Affiliation:
Division of Food Science and Biotechnology, Graduate School of Agriculture, Kyoto University, Uji, Kyoto 611-0011, Japan.