Structure-reactivity correlations and kinetic isotope effects in aromatic amine dehydrogenase
Hothi, P., Roujeinikova, A., Sutcliffe, M.J., Cullis, P., Leys, D., Scrutton, N.S.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Aromatic amine dehydrogenase, small subunit | A [auth D], B [auth H] | 122 | Alcaligenes faecalis | Mutation(s): 1 EC: 1.4.99.4 | 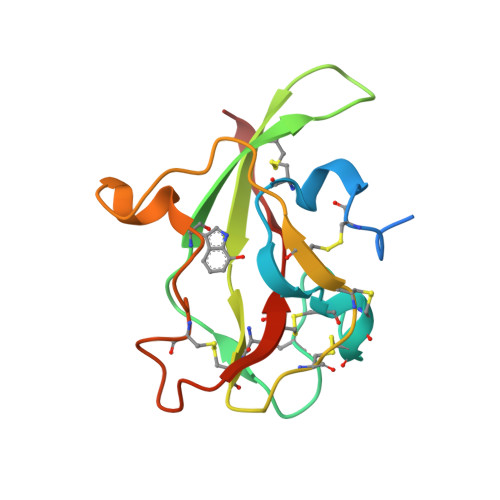 |
UniProt | |||||
Find proteins for P84887 (Alcaligenes faecalis) Explore P84887 Go to UniProtKB: P84887 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P84887 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Aromatic amine dehydrogenase, large subunit | C [auth A], D [auth B] | 362 | Alcaligenes faecalis | Mutation(s): 0 Gene Names: aauB EC: 1.4.99.4 | 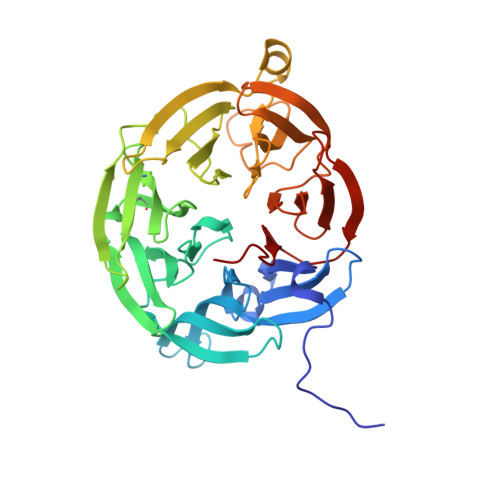 |
UniProt | |||||
Find proteins for P84888 (Alcaligenes faecalis) Explore P84888 Go to UniProtKB: P84888 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P84888 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ZHZ Query on ZHZ | F [auth H] | 2-(4-METHOXYPHENYL)ACETAMIDE C9 H11 N O2 OLKQIWCQICCYQS-UHFFFAOYSA-N |  | ||
| ZHH Query on ZHH | E [auth D] | 2-(4-METHOXYPHENYL)ETHANAMINE C9 H13 N O LTPVSOCPYWDIFU-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| TRQ Query on TRQ | A [auth D], B [auth H] | L-PEPTIDE LINKING | C11 H10 N2 O4 |  | TRP |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 70.848 | α = 90 |
| b = 88.813 | β = 90.39 |
| c = 80.044 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| MOSFLM | data reduction |
| SCALA | data scaling |