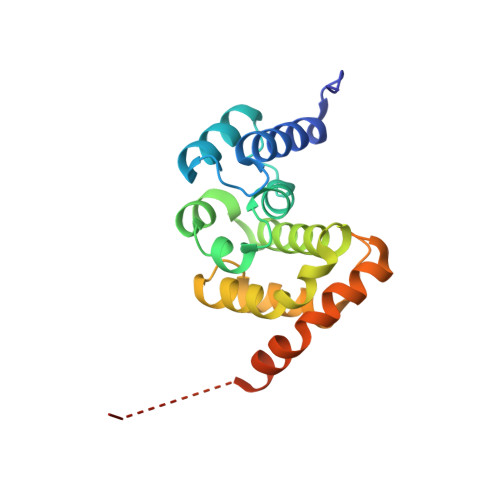
Crystal Structure of the C-terminal Domain of S.cerevisiae eIF5
Wei, Z., Xue, Y., Xu, H., Gong, W.(2006) J Mol Biol 359: 1-9
- PubMed: 16616930
- DOI: https://doi.org/10.1016/j.jmb.2006.03.037
- Primary Citation of Related Structures:
2FUL - PubMed Abstract:
eIF5, a GTPase-activating protein (GAP) specific for eIF2, plays a critical role in pre-initiation complex assembly and correct AUG selection during eukaryotic translation initiation. eIF5 is involved in the formation of the multifactor complex (MFC), an important intermediate of the 43S pre-initiation complex. The C-terminal domain (CTD) of eIF5 functions as the structural core in the MFC assembly. Here we report the 1.5A crystal structure of eIF5-CTD, confirming that eIF5-CTD contains an atypical HEAT motif. In addition, analyzing the electrostatic potential and the distribution of conserved residues on the protein surface, we confirm and suggest some potential regions of interactions between eIF5-CTD and other eIFs. The structure of eIF5-CTD provides useful information in understanding the mechanism of the MFC assembly.
Organizational Affiliation:
National Laboratory of Biomacromolecules, USTC-IBP Joint Laboratory for Protein Sciences, Institute of Biophysics, Chinese Academy of Sciences, Beijing, People's Republic of China.