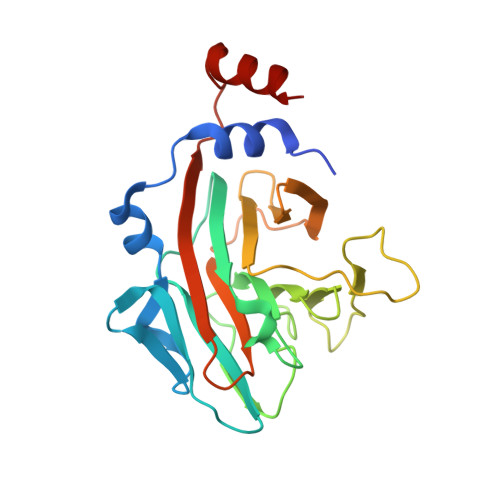
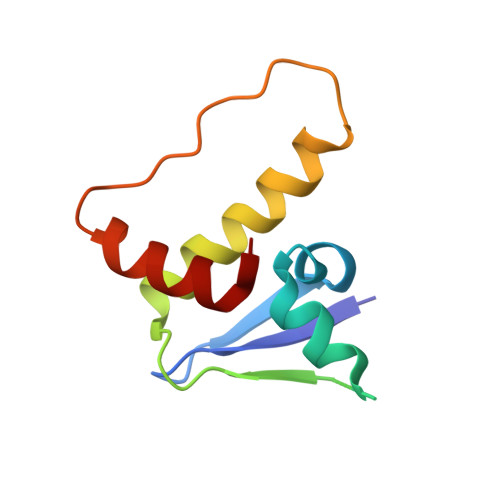
Structural and functional insights into the B30.2/SPRY domain
Woo, J.S., Imm, J.H., Min, C.K., Kim, K.J., Cha, S.S., Oh, B.H.(2006) EMBO J 25: 1353-1363
- PubMed: 16498413
- DOI: https://doi.org/10.1038/sj.emboj.7600994
- Primary Citation of Related Structures:
2FNJ - PubMed Abstract:
The B30.2/SPRY domain is present in approximately 700 eukaryotic (approximately 150 human) proteins, including medically important proteins such as TRIM5alpha and Pyrin. Nonetheless, the functional role of this modular domain remained unclear. Here, we report the crystal structure of an SPRY-SOCS box family protein GUSTAVUS in complex with Elongins B and C, revealing a highly distorted two-layered beta-sandwich core structure of its B30.2/SPRY domain. Ensuing studies identified one end of the beta-sandwich as the surface interacting with an RNA helicase VASA with a 40 nM dissociation constant. The sequence variation in TRIM5alpha responsible for HIV-1 restriction and most of the mutations in Pyrin causing familial Mediterranean fever map on this surface, implicating the corresponding region in many B30.2/SPRY domains as the ligand-binding site. The amino acids lining the binding surface are highly variable among the B30.2/SPRY domains, suggesting that these domains are protein-interacting modules, which recognize a specific individual partner protein rather than a consensus sequence motif.
Organizational Affiliation:
Division of Molecular and Life Sciences, Department of Life Sciences, Center for Biomolecular Recognition, Pohang University of Science and Technology, Pohang, Kyungbuk, Korea.