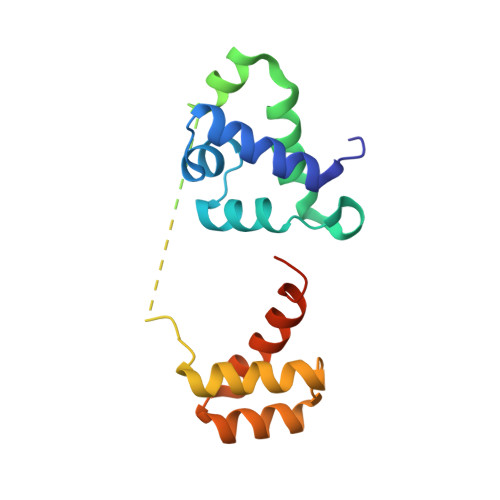
DNA recognition mechanism of the ONECUT homeodomain of transcription factor HNF-6
Iyaguchi, D., Yao, M., Watanabe, N., Nishihira, J., Tanaka, I.(2007) Structure 15: 75-83
- PubMed: 17223534
- DOI: https://doi.org/10.1016/j.str.2006.11.004
- Primary Citation of Related Structures:
2D5V - PubMed Abstract:
Hepatocyte nuclear factor-6 (HNF-6), a liver-enriched transcription factor, controls the development of various tissues, such as the pancreas and liver, and regulates the expression of several hepatic genes. This protein belongs to the ONECUT class of homeodomain proteins and contains a bipartite DNA-binding domain composed of a single cut domain and a characteristic homeodomain. This transcription factor has two distinct modes of DNA binding and transcriptional activation that use different coactivators depending on the target gene. The crystal structure of the bipartite DNA-binding domain of HNF-6alpha complexed with the HNF-6-binding site of the TTR promoter revealed the DNA recognition mechanism of this protein. Comparing our structure with the DNA-free structure of HNF-6 or the structure of Oct-1, we discuss characteristic features associated with DNA binding and the structural basis for the dual mode of action of this protein, and we suggest a strategy for variability of transcriptional activation of the target gene.
Organizational Affiliation:
Faculty of Advanced Life Sciences, Hokkaido University, Kita-10, Nishi-8, Sapporo 060-0810, Japan.