CU/ZN superoxide dismutase from neisseria meningitidis E73A mutant
DiDonato, M., Kassmann, C.J., Bruns, C.K., Cabelli, D.E., Cao, Z., Tabatabai, L.B., Kroll, J.S., Getzoff, E.D.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Superoxide dismutase [Cu-Zn] | 164 | Neisseria meningitidis | Mutation(s): 1 Gene Names: sodC EC: 1.15.1.1 | 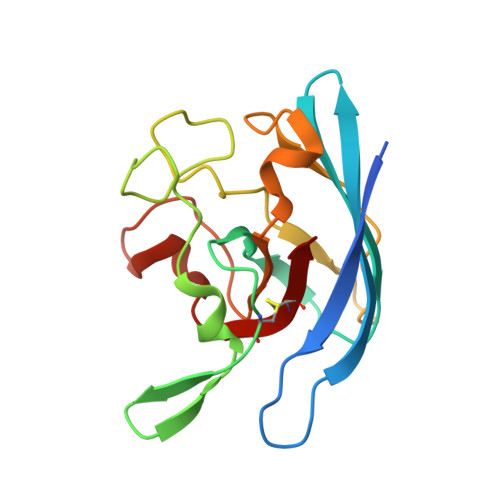 | |
UniProt | |||||
Find proteins for P57005 (Neisseria meningitidis serogroup A / serotype 4A (strain DSM 15465 / Z2491)) Explore P57005 Go to UniProtKB: P57005 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P57005 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ZN Query on ZN | D [auth A], M [auth B] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| CU1 Query on CU1 | C [auth A], E [auth A], L [auth B] | COPPER (I) ION Cu VMQMZMRVKUZKQL-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | F [auth A] G [auth A] H [auth A] I [auth A] J [auth A] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 145.874 | α = 90 |
| b = 48.697 | β = 109.57 |
| c = 49.042 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| AMoRE | phasing |
| SHELXL-97 | refinement |