Resolving the energy paradox of chaperone/usher-mediated fibre assembly
Zavialov, A.V., Tischenko, V.M., Fooks, L.J., Brandsdal, B.O., Aqvist, J., Zav'yalov, V.P., Macintyre, S., Knight, S.D.(2005) Biochem J 389: 685-694
- PubMed: 15799718
- DOI: https://doi.org/10.1042/BJ20050426
- Primary Citation of Related Structures:
1Z9S - PubMed Abstract:
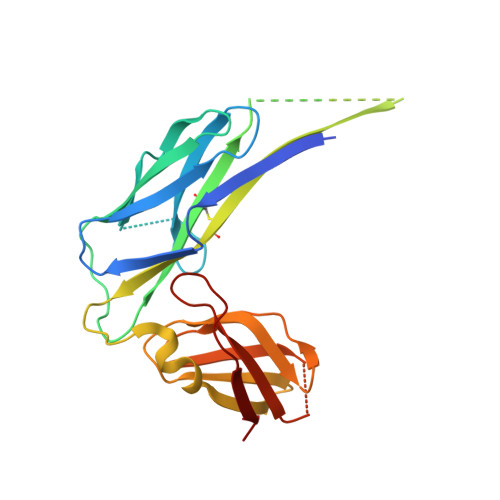
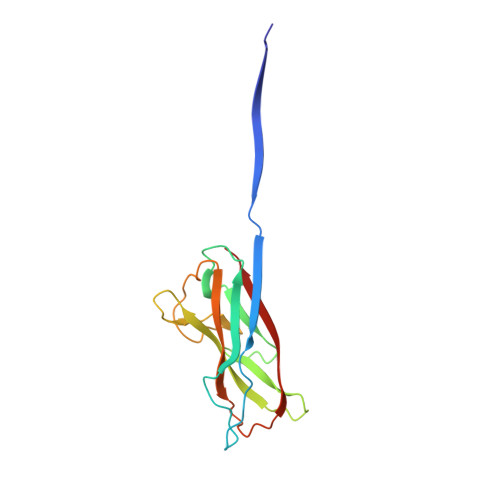
Periplasmic chaperone/usher machineries are used for assembly of filamentous adhesion organelles of Gram-negative pathogens in a process that has been suggested to be driven by folding energy. Structures of mutant chaperone-subunit complexes revealed a final folding transition (condensation of the subunit hydrophobic core) on the release of organelle subunit from the chaperone-subunit pre-assembly complex and incorporation into the final fibre structure. However, in view of the large interface between chaperone and subunit in the pre-assembly complex and the reported stability of this complex, it is difficult to understand how final folding could release sufficient energy to drive assembly. In the present paper, we show the X-ray structure for a native chaperone-fibre complex that, together with thermodynamic data, shows that the final folding step is indeed an essential component of the assembly process. We show that completion of the hydrophobic core and incorporation into the fibre results in an exceptionally stable module, whereas the chaperone-subunit pre-assembly complex is greatly destabilized by the high-energy conformation of the bound subunit. This difference in stabilities creates a free energy potential that drives fibre formation.
Organizational Affiliation:
Department of Molecular Biology, Uppsala Biomedical Center, Swedish University of Agricultural Sciences, Box 590, SE-753 24 Uppsala, Sweden. anton.zavialov@molbio.slu.se