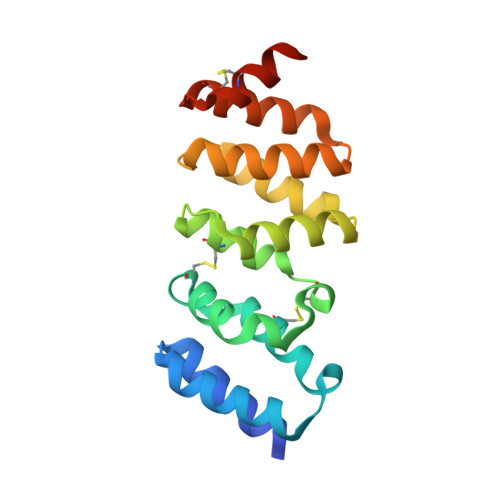
The first crystal structure of an archaeal helical repeat protein.
Yoneda, K., Sakuraba, H., Tsuge, H., Katunuma, N., Kuramitsu, S., Kawabata, T., Ohshima, T.(2005) Acta Crystallogr Sect F Struct Biol Cryst Commun 61: 636-639
- PubMed: 16511116
- DOI: https://doi.org/10.1107/S1744309105019263
- Primary Citation of Related Structures:
1WY6 - PubMed Abstract:
The crystal structure of ST1625p, a protein encoded by a hypothetical open reading frame ST1625 in the genome of the hyperthermophilic archaeon Sulfolobus tokodaii, was determined at 2.2 A resolution. The only sequence similarity exhibited by the amino-acid sequence of ST1625p was a 33% identity with the sequence of SSO0983p from S. solfataricus. The 19 kDa monomeric protein was observed to consist of a right-handed superhelix assembled from a tandem repeat of ten alpha-helices. A structural homology search using the DALI and MATRAS algorithms indicates that this protein can be classified as a helical repeat protein.
Organizational Affiliation:
Department of Biological Science and Technology, Faculty of Engineering, University of Tokushima, Tokushima 770-8506, Japan.