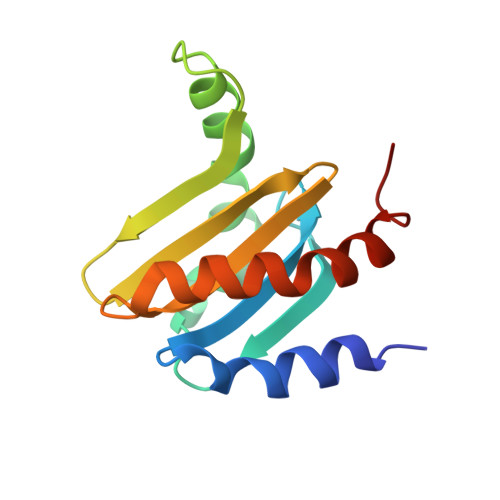
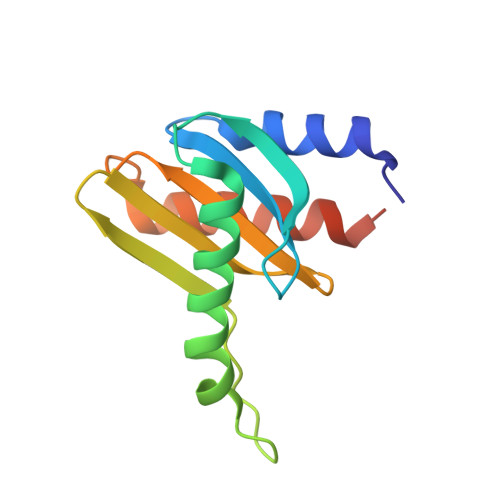
Crystal structure of the p14/MP1 scaffolding complex: How a twin couple attaches mitogen- activated protein kinase signaling to late endosomes
Kurzbauer, R., Teis, D., De Araujo, M.E., Maurer-Stroh, S., Eisenhaber, F., Bourenkov, G.P., Bartunik, H.D., Hekman, M., Rapp, U.R., Huber, L.A., Clausen, T.(2004) Proc Natl Acad Sci U S A 101: 10984-10989
- PubMed: 15263099
- DOI: https://doi.org/10.1073/pnas.0403435101
- Primary Citation of Related Structures:
1VET, 1VEU - PubMed Abstract:
Signaling pathways in eukaryotic cells are often controlled by the formation of specific signaling complexes, which are coordinated by scaffold and adaptor proteins. Elucidating their molecular architecture is essential to understand the spatial and temporal regulation of cellular signaling. p14 and MP1 form a tight (K(d) = 12.8 nM) endosomal adaptor/scaffold complex, which regulates mitogen-activated protein kinase (MAPK) signaling. Here, we present the 1.9-A crystal structure of a biologically functional p14/MP1 complex. The overall topology of the individual MP1 and p14 proteins is almost identical, having a central five-stranded beta-sheet sandwiched between a two-helix and a one-helix layer. Formation of the p14/MP1 heterodimer proceeds by beta-sheet augmentation and yields a unique, almost symmetrical, complex with several potential protein-binding sites on its surface. Mutational analysis allowed identification of the p14 endosomal adaptor motif, which seems to orient the complex relative to the endosomal membrane. Two highly conserved and hydrophobic protein-binding sites are located on the opposite "cytoplasmic" face of the p14/MP1 heterodimer and might therefore function as docking sites for the target proteins extracellular regulated kinase (ERK) 1 and MAPK/ERK kinase 1. Furthermore, detailed sequence analyses revealed that MP1/p14, together with profilins, define a protein superfamily of small subcellular adaptor proteins, named ProflAP. Taken together, the presented work provides insight into the spatial regulation of MAPK signaling, illustrating how p14 and MP1 collaborate as an endosomal adaptor/scaffold complex.
Organizational Affiliation:
Institute for Molecular Pathology, Dr. Bohr-Gasse 7, A-1030 Vienna, Austria.