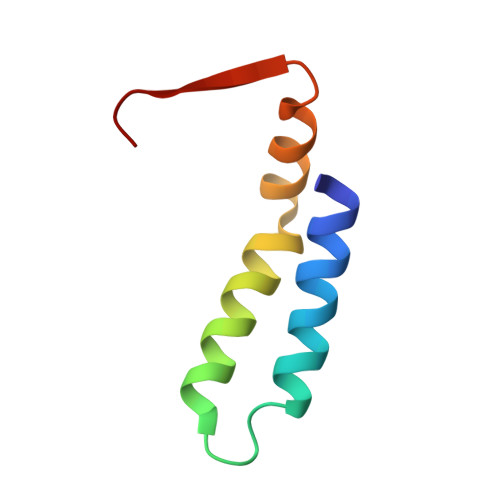
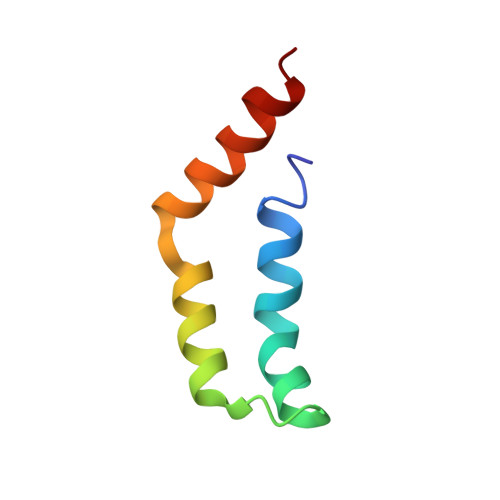
The tetrameric L27 domain complex as an organization platform for supramolecular assemblies
Feng, W., Long, J.-F., Fan, J.-S., Suetake, T., Zhang, M.(2004) Nat Struct Mol Biol 11: 475-480
- PubMed: 15048107
- DOI: https://doi.org/10.1038/nsmb751
- Primary Citation of Related Structures:
1RSO - PubMed Abstract:
L27 domain, initially identified in the Caenorhabditis elegans Lin-2 and Lin-7 proteins, is a protein interaction module that exists in a large family of scaffold proteins. The domain can function as an organization center of large protein assemblies required for establishment and maintenance of cell polarity. We have solved the high-resolution NMR structure of a tetrameric complex of L27 domains containing two SAP97-mLin-2 L27 domain heterodimers. Each L27 domain contains three a-helices. The first two helices of each domain are packed together to form a four-helical bundle in the heterodimer. The third helix of each L27 domain forms another four-helical bundle that assembles the two heterodimers into a tetramer. The structure of the complex provides a mechanistic explanation for L27 domain-mediated polymerization of scaffold proteins, a process that is crucial for the assembly of supramolecular complexes in asymmetric cells.
Organizational Affiliation:
Department of Biochemistry, Molecular Neuroscience Center, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China.