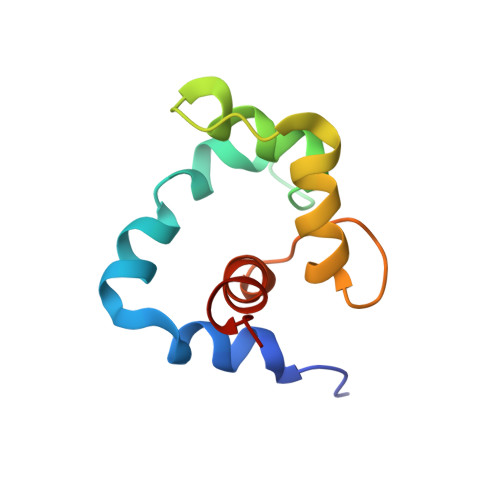
NMR Structure of a Bifunctional Rhodamine Labeled N-Domain of Troponin C Complexed with the Regulatory "Switch" Peptide from Troponin I: Implications for in Situ Fluorescence Studies in Muscle Fibers
Mercier, P., Ferguson, R.E., Irving, M., Corrie, J.E.T., Trentham, D.R., Sykes, B.D.(2003) Biochemistry 42: 4333-4348
- PubMed: 12693929
- DOI: https://doi.org/10.1021/bi027041n
- Primary Citation of Related Structures:
1NPQ - PubMed Abstract:
The structure of the calcium-saturated regulatory domain of skeletal troponin C (sNTnC) complexed with the switch peptide comprising residues 115-131 of troponin I (TnI), and with a bifunctional rhodamine fluorescent label attached to residues 56 (E56C) and 63 (E63C) on the C helix of sNTnC, has been determined using nuclear magnetic resonance (NMR) spectroscopy. The structure shows that the integrity of the C helix is not altered by the E(56,63)C mutations or by the presence of the bifunctional rhodamine and that the label does not interact with the hydrophobic cleft of sNTnC. Moreover, the overall fold of the protein and the position of the TnI peptide are similar to those observed previously with related cardiac NTnC complexes with residues 147-163 of cardiac TnI [Li et al. (1999) Biochemistry 38, 8289-8298] and including the drug bepridil [Wang et al. (2002) J. Biol. Chem. 277, 31124-31133]. The degree of opening of the structure is reduced as compared to that of calcium-saturated sNTnC in the absence of the switch peptide [Gagné et al. (1995) Nat. Struct. Biol. 2, 784-789]. The switch peptide is bound in a shallow and complementary hydrophobic surface cleft largely defined by helices A and B and also has key ionic interactions with sNTnC. These results show that bifunctional rhodamine probes can be attached to surface helices via suitable pairs of solvent-accessible residues that have been mutated to cysteines, without altering the conformation of the labeled domain. A set of such probes can be used to determine the orientation and motion of the target domain in the cellular environment [Corrie et al. (1999) Nature 400, 425-430; Ferguson et al. (2003) Mol. Cell 11(4), in press].
Organizational Affiliation:
CIHR Group in Protein Structure and Function, Department of Biochemistry, University of Alberta, Edmonton, Alberta, Canada T6G 2H7.