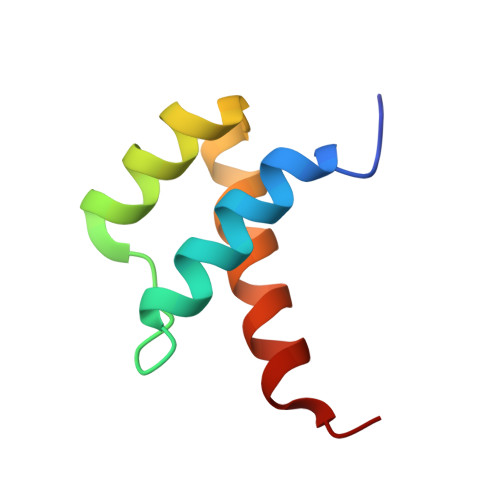
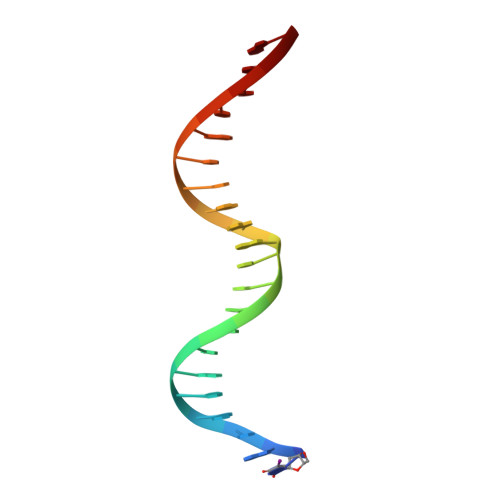
A Hoogsteen base pair embedded in undistorted B-DNA
Aishima, J., Gitti, R.K., Noah, J.E., Gan, H.H., Schlick, T., Wolberger, C.(2002) Nucleic Acids Res 30: 5244-5252
- PubMed: 12466549
- DOI: https://doi.org/10.1093/nar/gkf661
- Primary Citation of Related Structures:
1K61 - PubMed Abstract:
Hoogsteen base pairs within duplex DNA typically are only observed in regions containing significant distortion or near sites of drug intercalation. We report here the observation of a Hoogsteen base pair embedded within undistorted, unmodified B-DNA. The Hoogsteen base pair, consisting of a syn adenine base paired with an anti thymine base, is found in the 2.1 A resolution structure of the MATalpha2 homeodomain bound to DNA in a region where a specifically and a non-specifically bound homeodomain contact overlapping sites. NMR studies of the free DNA show no evidence of Hoogsteen base pair formation, suggesting that protein binding favors the transition from a Watson-Crick to a Hoogsteen base pair. Molecular dynamics simulations of the homeodomain-DNA complex support a role for the non-specifically bound protein in favoring Hoogsteen base pair formation. The presence of a Hoogsteen base pair in the crystal structure of a protein-DNA complex raises the possibility that Hoogsteen base pairs could occur within duplex DNA and play a hitherto unrecognized role in transcription, replication and other cellular processes.
Organizational Affiliation:
Department of Biophysics and Biophysical Chemistry, Johns Hopkins University School of Medicine, Baltimore, MD 21205-2185, USA.