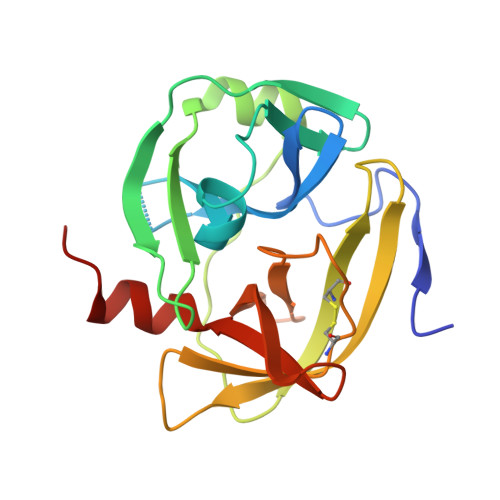
Crystal structure of the serine protease domain of Sesbania mosaic virus polyprotein and mutational analysis of residues forming the S1-binding pocket
Gayathri, P., Satheshkumar, P.S., Prasad, K., Nair, S., Savithri, H.S., Murthy, M.R.N.(2006) Virology 346: 440-451
- PubMed: 16356524
- DOI: https://doi.org/10.1016/j.virol.2005.11.011
- Primary Citation of Related Structures:
1ZYO - PubMed Abstract:
Sesbania mosaic virus (SeMV) polyprotein is processed by its N-terminal serine protease domain. The crystal structure of the protease domain was determined to a resolution of 2.4 A using multiple isomorphous replacement and anomalous scattering. The SeMV protease domain exhibited the characteristic trypsin fold and was found to be closer to cellular serine proteases than to other viral proteases. The residues of the S1-binding pocket, H298, T279 and N308 were mutated to alanine in the DeltaN70-Protease-VPg polyprotein, and the cis-cleavage activity was examined. The H298A and T279A mutants were inactive, while the N308A mutant was partially active, suggesting that the interactions of H298 and T279 with P1-glutamate are crucial for the E-T/S cleavage. A region of exposed aromatic amino acids, probably essential for interaction with VPg, was identified on the protease domain, and this interaction could play a major role in modulating the function of the protease.
Organizational Affiliation:
Molecular Biophysics Unit, Indian Institute of Science, Bangalore 560 012, India.