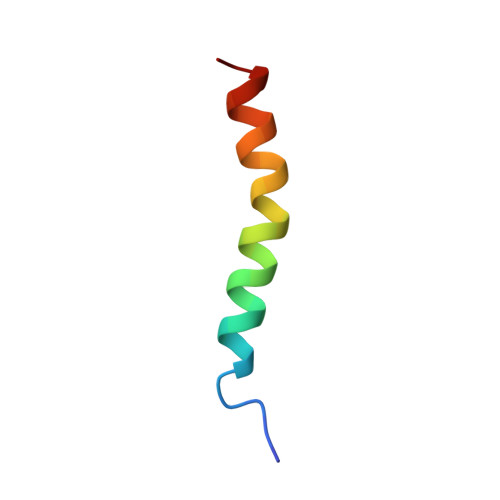
NMR structure of the glucose-dependent insulinotropic polypeptide fragment, GIP(1-30)amide.
Alana, I., Hewage, C.M., Malthouse, J.P.G., Parker, J.C., Gault, V.A., O'Harte, F.P.M.(2004) Biochem Biophys Res Commun 325: 281-286
- PubMed: 15522230
- DOI: https://doi.org/10.1016/j.bbrc.2004.10.033
- Primary Citation of Related Structures:
1T5Q - PubMed Abstract:
Glucose-dependent insulinotropic polypeptide is an incretin hormone that stimulates insulin secretion and reduces postprandial glycaemic excursions. The glucose-dependent action of GIP on pancreatic beta-cells has attracted attention towards its exploitation as a potential drug for type 2 diabetes. Use of NMR or X-ray crystallography is vital to determine the three-dimensional structure of the peptide. Therefore, to understand the basic structural requirements for the biological activity of GIP, the solution structure of the major biologically active fragment, GIP(1-30)amide, was investigated by proton NMR spectroscopy and molecular modelling. The structure is characterised by a full length alpha-helical conformation between residues F(6) and A(28). This structural information could play an important role in the design of therapeutic agents based upon GIP receptor agonists.
Organizational Affiliation:
Department of Biochemistry, Centre for Synthesis and Chemical Biology, Conway Institute of Biomolecular and Biomedical Research, University College Dublin, Belfield, Dublin 4, Ireland.