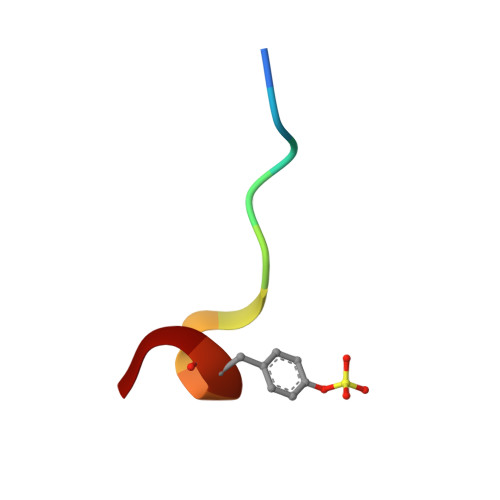
Discovery and evaluation of potent P1 aryl heterocycle-based thrombin inhibitors
Young, M.B., Barrow, J.C., Glass, K.L., Lundell, G.F., Newton, C.L., Pellicore, J.M., Rittle, K.E., Selnick, H.G., Stauffer, K.J., Vacca, J.P., Williams, P.D., Bohn, D., Clayton, F.C., Cook, J.J., Krueger, J.A., Kuo, L.C., Lewis, S.D., Lucas, B.J., McMasters, D.R., Miller-Stein, C., Pietrak, B.L.(2004) J Med Chem 47: 2995-3008
- PubMed: 15163182
- DOI: https://doi.org/10.1021/jm030303e
- Primary Citation of Related Structures:
1SL3 - PubMed Abstract:
In an effort to discover potent, clinically useful thrombin inhibitors, a rapid analogue synthetic approach was used to explore the P(1) region. Various benzylamines were coupled to a pyridine/pyrazinone P(2)-P(3) template. One compound with an o-thiadiazole benzylic substitution was found to have a thrombin K(i) of 0.84 nM. A study of ortho-substituted five-membered-ring heterocycles was undertaken and subsequently demonstrated that the o-triazole and tetrazole rings were optimal. Combination of these potent P(1) aryl heterocycles with a variety of P(2)-P(3) groups produced a compound with an extraordinary thrombin inhibitory activity of 1.4 pM. It is hoped that this potency enhancement in P(1) will allow for more diversification in the P(2)-P(3) region to ultimately address additional pharmacological concerns.
Organizational Affiliation:
Medicinal Chemistry, Merck Research Laboratories, Merck and Co., Inc., WP14-3, Post Office Box 4, Sumneytown Pike, West Point, PA 19486, USA. mary_beth_young@merck.com