Crystal Structures of the Quinone Oxidoreductase from Thermus thermophilus HB8 and Its Complex with NADPH: Implication for NADPH and Substrate Recognition
Shimomura, Y., Kakuta, Y., Fukuyama, K.(2003) J Bacteriol 185: 4211-4218
- PubMed: 12837796
- DOI: https://doi.org/10.1128/JB.185.14.4211-4218.2003
- Primary Citation of Related Structures:
1IYZ, 1IZ0 - PubMed Abstract:
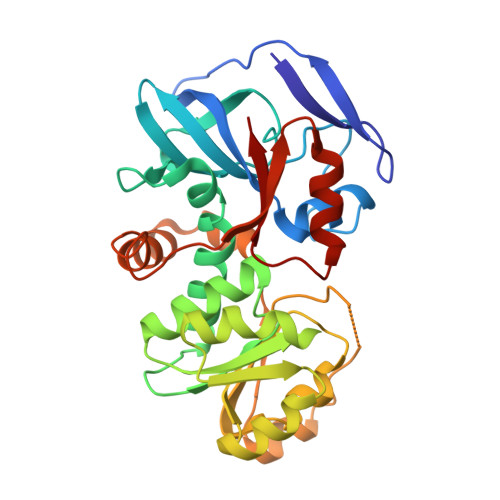
The crystal structures of the zeta-crystalline-like soluble quinone oxidoreductase from Thermus thermophilus HB8 (QOR(Tt)) and of its complex with NADPH have been determined at 2.3- and 2.8-A resolutions, respectively. QOR(Tt) is composed of two domains, and its overall fold is similar to the folds of Escherichia coli quinone oxidoreductase (QOR(Ec)) and horse liver alcohol dehydrogenase. QOR(Tt) forms a homodimer in the crystal by interaction of the betaF-strands in domain II, forming a large beta-sheet that crosses the dimer interface. High thermostability of QOR(Tt) was evidenced by circular dichroic measurement. NADPH is located between the two domains in the QOR(Tt)-NADPH complex. The disordered segment involved in the coenzyme binding of apo-QOR(Tt) becomes ordered upon NADPH binding. The segment covers an NADPH-binding cleft and may serve as a lid. The 2'-phosphate group of the adenine of NADPH is surrounded by polar and positively charged residues in QOR(Tt), suggesting that QOR(Tt) binds NADPH more readily than NADH. The putative substrate-binding site of QOR(Tt), unlike that of QOR(Ec), is largely blocked by nearby residues, permitting access only to small substrates. This may explain why QOR(Tt) has weak p-benzoquinone reduction activity and is inactive with such large substrates of QOR(Ec) as 5-hydroxy-1,4-naphthoquinone and phenanthraquinone.
Organizational Affiliation:
Department of Biology, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan.