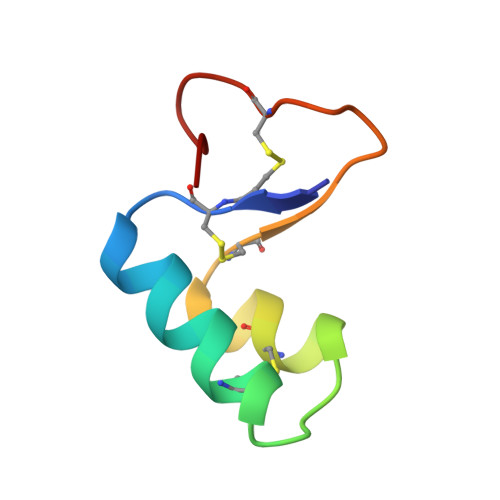
NMR structural determination of viscotoxin A3 from Viscum album L.
Romagnoli, S., Ugolini, R., Fogolari, F., Schaller, G., Urech, K., Giannattasio, M., Ragona, L., Molinari, H.(2000) Biochem J 350: 569-577
- PubMed: 10947973
- Primary Citation of Related Structures:
1ED0 - PubMed Abstract:
The high-resolution three-dimensional structure of the plant toxin viscotoxin A3, from Viscum album L., has been determined in solution by (1)H NMR spectroscopy at pH 3.6 and 12 degrees C (the structure has been deposited in the Protein Data Bank under the id. code 1ED0). Experimentally derived restraints including 734 interproton distances from nuclear Overhauser effect measurements, 22 hydrogen bonds, 32 φ angle restraints from J coupling measurements, together with three disulphide bridge constraints were used as input in restrained molecular dynamics, followed by minimization, using DYANA and Discover. Backbone and heavy atom root-mean-square deviations were 0.47+/-0.11 A (1 A=10(-10) m) and 0.85+/-0.13 A respectively. Viscotoxin A3 consists of two alpha-helices connected by a turn and a short stretch of antiparallel beta-sheet. This fold is similar to that found in other thionins, such as crambin, hordothionin-alpha and -beta, phoratoxin A and purothionin-alpha and -beta. The difference in the observed biological activity for thionins of known structure is discussed in terms of the differences in the calculated surface potential distribution, playing an important role in their function through disruption of cell membranes. In addition, the possible role in DNA binding of the helix-turn-helix motif of viscotoxin A3 is discussed.
Organizational Affiliation:
Dipartimento Scientifico e Tecnologico, Cà Vignal 1, Strada le Grazie, 37134 Verona, Italy.