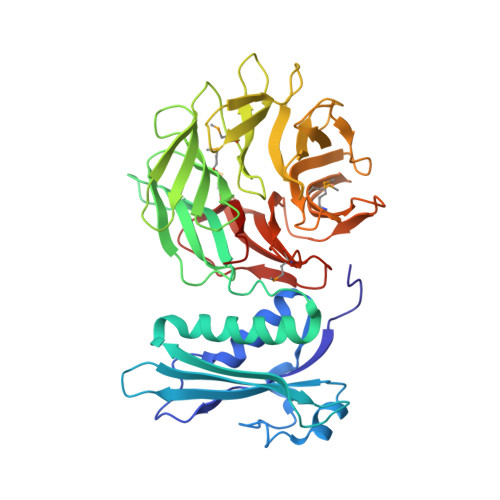
Structure of the Escherichia coli TolB protein determined by MAD methods at 1.95 A resolution.
Abergel, C., Bouveret, E., Claverie, J.M., Brown, K., Rigal, A., Lazdunski, C., Benedetti, H.(1999) Structure 7: 1291-1300
- PubMed: 10545334
- DOI: https://doi.org/10.1016/s0969-2126(00)80062-3
- Primary Citation of Related Structures:
1CRZ - PubMed Abstract:
The periplasmic protein TolB from Escherichia coli is part of the Tol-PAL (peptidoglycan-associated lipoprotein) multiprotein complex used by group A colicins to penetrate and kill cells. TolB homologues are found in many gram-negative bacteria and the Tol-PAL system is thought to play a role in bacterial envelope integrity. TolB is required for lethal infection by Salmonella typhimurium in mice.
Organizational Affiliation:
Information Génétique et Structurale, CNRS-UMR 1889 Institut de Biologie Structurale et Microbiologie 31 Chemin Joseph Aiguier, Marseille, 13402, Cedex 20, France. chantal@igs.cnrs-mrs.fr